Session Information
Date: Saturday, November 16, 2024
Title: Abstracts: Miscellaneous Rheumatic & Inflammatory Diseases I
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Susac syndrome (SuS) is an exceedingly rare microvasculopathy of the brain, retina and inner ear affecting young patients with potential lifelong sequelae. No randomized controlled trial has been published or declared as ongoing in SuS. We aimed to compare the efficacy of steroids given alone or associated with immunosuppressive (IS) agents and/or intravenous immunoglobulin (IVIg) for the prevention of relapse in SuS.
Methods: CarESS study is a prospective multicenter national cohort study that started in December 2011, including all consecutive patients with SuS. SuS was defined either by i) the triad of encephalopathy with typical brain MRI abnormalities, cochleo-vestibular damage and multiple occlusions of retinal central artery branches or ii) at least two of the three criteria without any alternative diagnosis. Collected data included fundoscopy, retinal angiography, audiometry, cerebrospinal fluid, brain MRI and treatment received. The primary outcome was the first relapse defined by new clinical symptoms or signs and new abnormalities on retinal angiography, audiometry or brain MRI leading to treatment intensification.
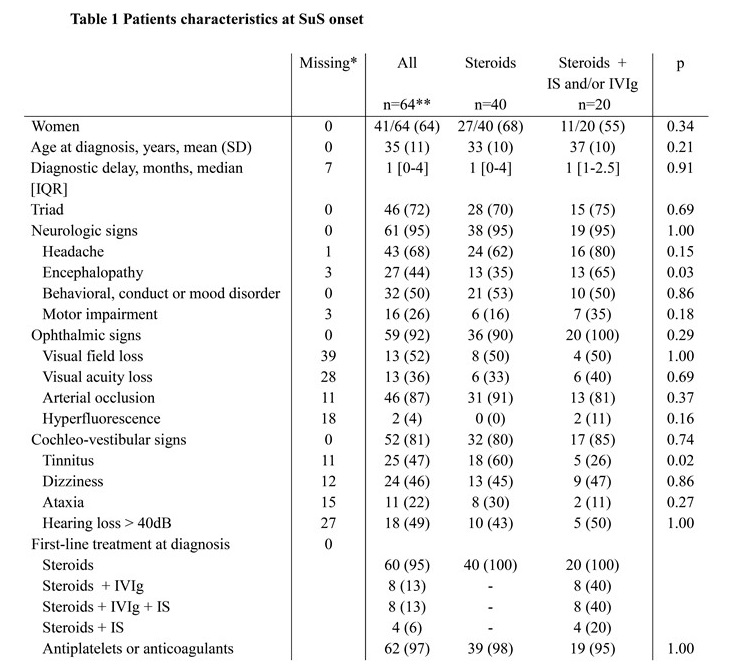
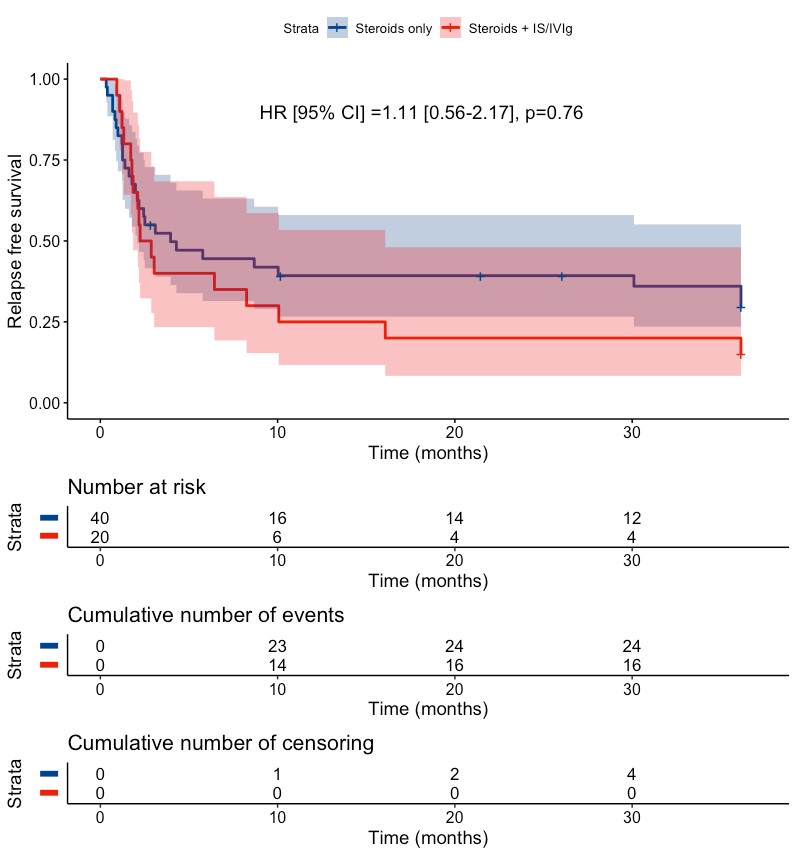
Results: Sixty-four SuS patients (mean (SD) age at diagnosis 35 (11) years; female ratio 64%) were included. Among them, 40 patients received steroids alone as a first-line therapy while 20 received steroids in combination with IS agents and/or IVIg. Overall, 46 (72%) patients experienced a first relapse with a median [95%CI] relapse-free survival time of 3.96 [2.24-16.07] months. Comparison of relapse-free survival demonstrated no significant difference between the two treatment strategies (HR [95%CI] = 1.11 [0.56-2.17], p=0.76). In patients who first relapsed while treated by steroids alone, there was no significant difference in second relapse-free survival between those who did or did not receive IS agents and/or IVIg as a second-line therapy (HR [95%CI] = 2.66 [0.63-11.18], p = 0.18).
Conclusion: Our findings did not support the systematic use of IS drugs in SuS to prevent relapse. Immunosuppressive drugs should be tested in a multicenter prospective randomized trial in SuS with the primary aim to prevent irreversible damage
Qualitative variables are expressed as a number (percentage). IS, immunosuppressive; IVIg, intravenous immunoglobulin.; dB, decibels. Immunosuppressive drugs included cyclophosphamide (n=9), azathioprine (n=4) or mycophenolate mofetil (n=1)
*at start of treatment
** 4 patients received antiplatelets or anticoagulants only (i.e. without steroids nor IS/IVIg)
Kaplan-Meier curves of the survival without first relapse among patients who did (steroids + IS and/or IVIg) or did not (steroids) receive IS agents and/or IVIg.
P value was calculated with a Cox proportional hazard model. The model was adjusted on age, sex, center and presence of encephalopathy at diagnosis.
Patients who did not received either steroids, IS agents or IVIg at disease onset (n=4) were excluded from the analysis. The shaded areas refer to de 95% confidence bands. The “Cumulative Number of Censoring” refers to patients who were lost to follow-up.
To cite this abstract in AMA style:
Kachaner A, Mageau A, Goulenok T, Francois C, Chauveheid M, Papo T, Sacré K. Addition of Immunosuppressive Agents And/or Intravenous Immunoglobulin to Steroids in the Treatment of Susac Syndrome: A National Cohort Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/addition-of-immunosuppressive-agents-and-or-intravenous-immunoglobulin-to-steroids-in-the-treatment-of-susac-syndrome-a-national-cohort-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/addition-of-immunosuppressive-agents-and-or-intravenous-immunoglobulin-to-steroids-in-the-treatment-of-susac-syndrome-a-national-cohort-study/