Session Information
Date: Monday, November 18, 2024
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The latest European and American recommendations support tapering of biological DMARDs (bDMARDs) in patients with persistent remission or low disease activity. Most clinicians use disease activity scores to monitor dose tapering. Considering the inter-individual variation in serum concentration of biologics, this trial-and-error approach is inevitably associated with increased risk of flare. Reducing bDMARD doses in patients with low serum concentrations can lead to ineffective drug levels and subsequent flares. There is strong evidence that withdrawal of bDMARDs result in flares. A novel approach involves a tailored tapering strategy based on serum concentration assessment, known as therapeutic drug monitoring (TDM). The concentration-effect curve of adalimumab (ADA) suggests that patients with levels above 5 mg/L are at risk of overexposure. An ADA concentration of 5 mg/L is likely necessary for an adequate clinical response in the initial treatment phase. However, to control disease activity after 28 weeks, lower concentrations might be sufficient. Our study aimed to assess whether a strategy using TDM-based dose adjustments to a target concentration of 2 mg/L was non-inferior to that with a concentration of 5 mg/L with respect to disease activity. Secondarily, superiority in reducing the amount of drug administered was assessed between both groups.
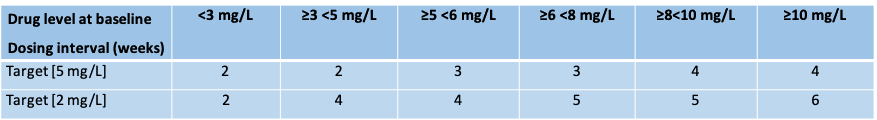
Methods: Sixty-two rheumatoid arthritis (RA) patients on ongoing ADA therapy (40 mg every other week) for at least 28 weeks, with low disease activity and an ADA concentration of > 5 mg/L were enrolled. Patients were randomly 1:1 assigned to dose reduction aiming a serum drug level of either 2 mg/L or 5 mg/L. The newly developed algorithm based on the PK/PD model of Ternant (1), determined the dosing interval to achieve the target drug level (Table 1). Clinical visits were scheduled at baseline (prior to dose reduction), 12 weeks and 24 weeks thereafter. The primary endpoint was the mean time weighted DAS28-CRP (MTW-DAS28) after 24 weeks. Noninferiority was defined as a difference with an upper 95%CI no greater than 0.6. Secondary outcomes included number of flares and ADA dosing, expressed as number of injections used during 24 weeks.
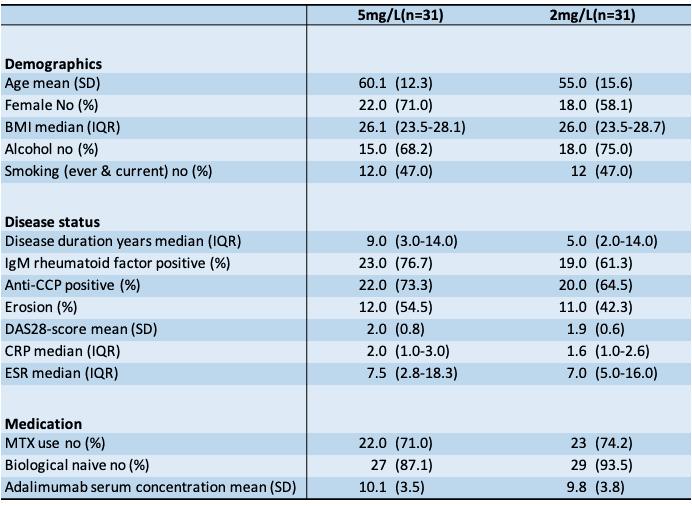
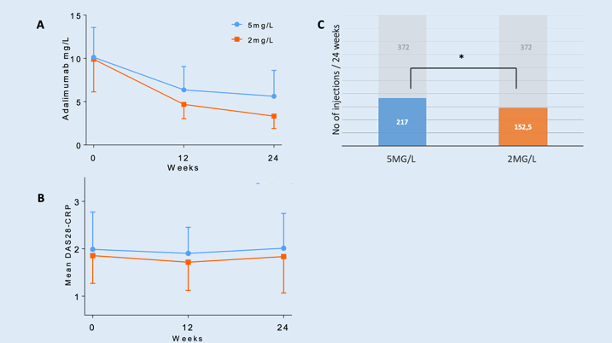
Results: Randomization created similar study groups (Table 2). The mean ADA concentration decreased from 10.1 mg/L (SD 3.5) to 5.6 mg/L (3.0) and from 9.9 mg/L (3.8) to 3.3 mg/L (1.4) respectively for the 5 mg/L and 2 mg/L group. The MTW-DAS28 at week 24 was 0.23 lower in the 2mg/L group [95%CI –0.07;0.53] P=0.14. The odds of having a flare were 1,30 higher in the 2mg/L group, which was a nonsignificant difference (OR 1.30 [95%CI 0.31;5.38] P=0.72). The total number of injections over 24 weeks was significantly lower in 2mg/L group: 30% difference (2 [95%CI –1,31; –2.85] P< 0.001).
Conclusion: TDM-based tapering strategy targeting a drug level of 2 mg/L is non-inferior to a target level of 5 mg/L with respect to disease activity and number of flares, while it saves a significant amount of medication. Moreover, it reduces patients’ burden of self-injections and costs.
References
1. Ternant D, Ducourau E, Fuzibet P, et al. British Journal of Clinical Pharmacology. 2015; 79(2):286-97
No number; SD standard deviation; ADA adalimumab; * significant difference.
To cite this abstract in AMA style:
Atiqi S, Wientjes M, Leeuw M, Boekel L, Hooijberg F, Loeff F, Krieckaert C, De Vries A, Nurmohamed M, Rispens T, Boers M, van den Bemt B, den Broeder A, Wolbink G. Adalimumab Dose Reduction Using Therapeutic Drug Monitoring to Manage Low Disease Activity in Rheumatoid Arthritis: A Single-Blind, Non-Inferiority, Randomized Clinical Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/adalimumab-dose-reduction-using-therapeutic-drug-monitoring-to-manage-low-disease-activity-in-rheumatoid-arthritis-a-single-blind-non-inferiority-randomized-clinical-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adalimumab-dose-reduction-using-therapeutic-drug-monitoring-to-manage-low-disease-activity-in-rheumatoid-arthritis-a-single-blind-non-inferiority-randomized-clinical-trial/