Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: In Denmark, mandatory switches between originators and corresponding biosimilar products have been performed for economic reasons for nearly a decade (1). Starting in January 2021, a biosimilar-to-biosimilar adalimumab switch from SB5 (Imraldi®) to GP2017 (Hyrimoz®) was performed in patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA). A subgroup of patients had previously made an additional adalimumab switch, from the originator (Humira®) to SB5 (November 2018). Evidence regarding biosimilar-to-biosimilar switch effects is scarce.
This study aims to explore GP2017 treatment retention following the SB5 switch in Danish patients with RA, PsA and axSpA, and the influence of previous originator adalimumab (ADAo) treatment history.
Methods: Observational cohort study based on prospectively registered data in the nationwide registry DANBIO (2). Patients with RA, PsA and axSpA who switched from SB5 to GP2017 in the time period January 1st 2021-June 30th 2021 were identified. Characteristics at GP2017 treatment start (baseline) were described. Follow-up was until one year after baseline, death or last visit in DANBIO, whichever came first. Flare rates 6 months before versus after the switch (RA and PsA: ΔDAS28 >1.2, axSpA: ΔASDAS >1.3) were inspected. One-year GP2017 retention was estimated with Kaplan-Meier curves (log-rank tests) according to previous ADAo treatment (yes/no). We performed Cox regression analyses stratified by diagnosis to identify characteristics associated with treatment retention. The analyses were adjusted for sex, baseline age, disease activity, patient global score on a VAS, disease duration, concomitant methotrexate, and previous ADAo history.
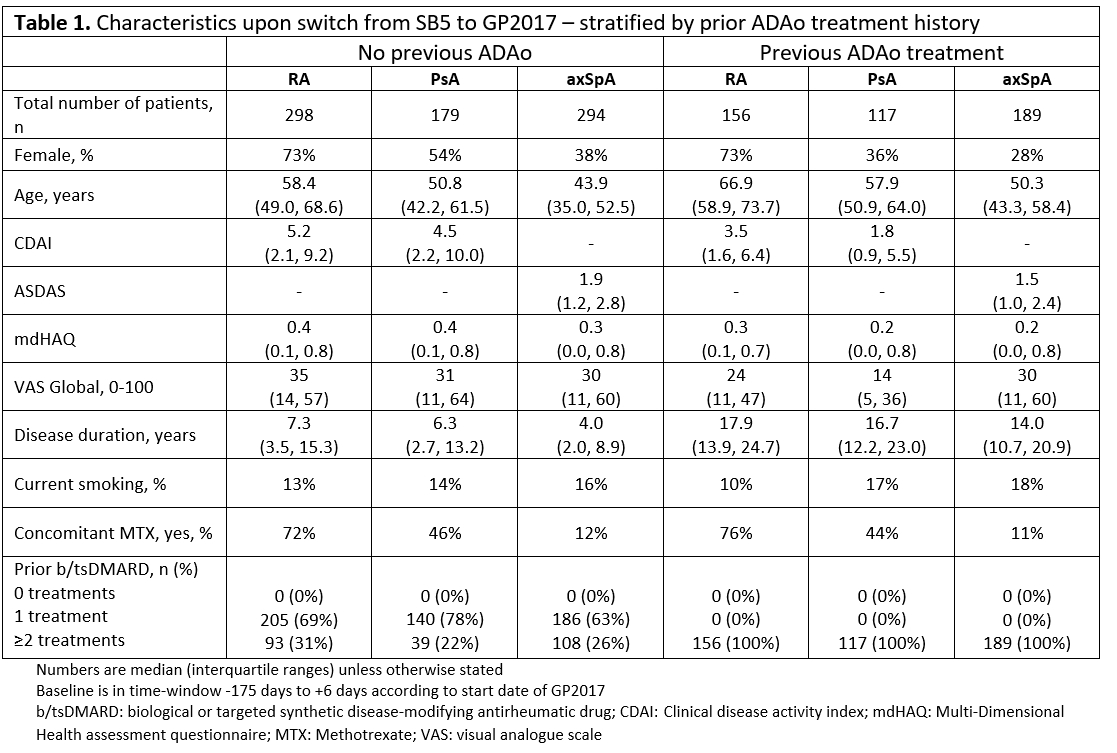
Results: Overall, 1233 patients (454 RA, 296 PsA, 483 axSpA) were included. At time of switch, patients with ADAo treatment history were older, had longer disease duration and lower disease activity than patients not previously treated with ADAo, while use of methotrexate was comparable for all diagnoses (Table 1).
Similar flare rates were observed for all diagnoses before versus after switch (details not shown).
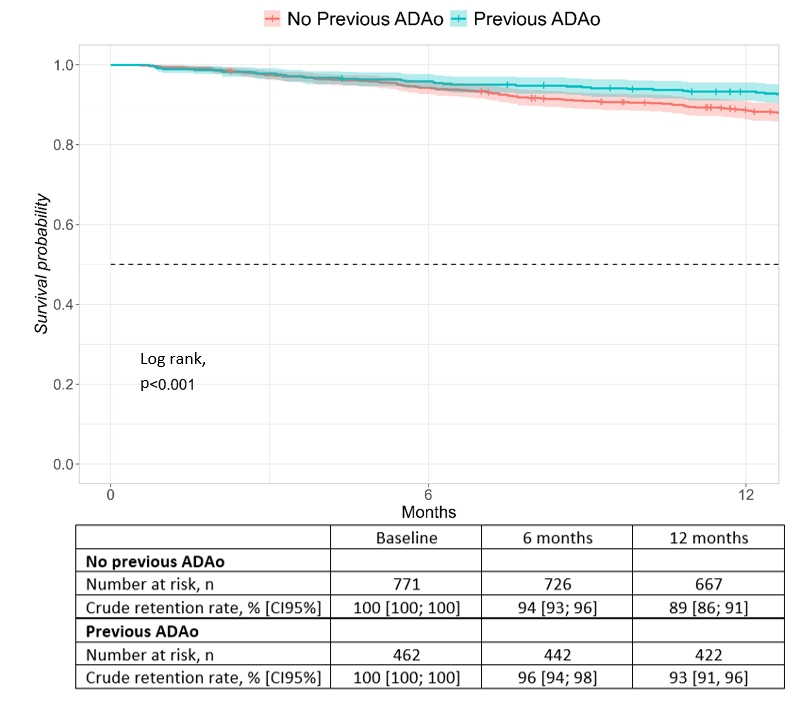
Patients previously treated with ADAo had higher GP2017 one-year retention rate than patients with no ADAo treatment history (93% and 89%, Figure 2). Stratified by diagnosis, this difference was only significant for RA (log rank test, p=0.028, details not shown).
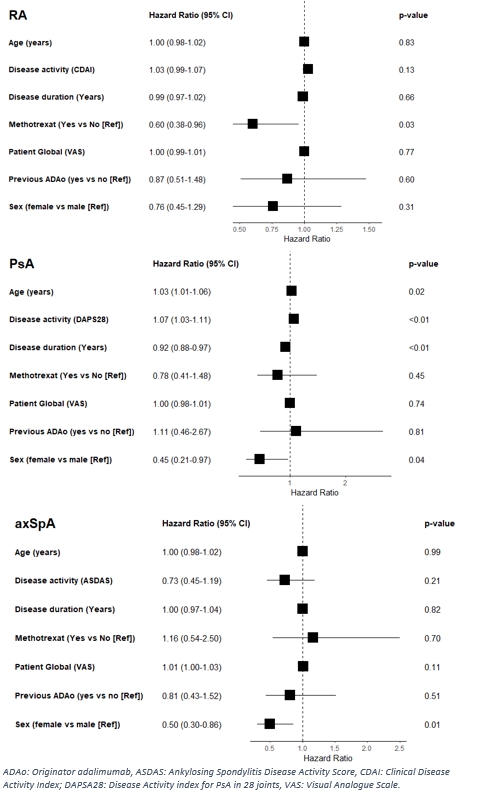
In Cox regression analyses, higher treatment retention rates were associated with methotrexate use (only RA) and male sex (PsA and axSpA), whereas there was no impact of ADAo history (Figure 2).
Conclusion: This nationwide observational study describes stable treatment outcomes following a mandatory switch from the adalimumab biosimilar SB5 to GP2017 – with GP2017 one-year retention rates of nearly 90% and unaffected flare rates. The impact of adalimumab originator treatment history was minor. These results support the use of biosimilar adalimumab in routine care.
References
- Nabi H, Hetland ML, Glintborg B et al. ARD 2021
- Ibfelt EH, et al. Clin Epidemiol. 2017
*MH and BG are shared last author.
Acknowledgements
Contributors to the DANBIO registry. Niels Steen Krogh, Zitelab, for datamanagement.
To cite this abstract in AMA style:
Jensen K, Nabi H, Jensen D, Pedersen J, Hendricks O, Loft A, Colic A, Danebod K, Kristensen S, Munk H, Lomborg N, Manilo N, Chrysidis S, Just S, Linauskas A, Højgaard P, Thygesen P, Kildemand M, Oestgaard R, Hetland M, Glintborg B. Adalimumab Biosimilar-to-biosimilar Switch in Patients with Inflammatory Rheumatic Diseases – Real-world Evidence from the Nationwide Danish Registry, DANBIO [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/adalimumab-biosimilar-to-biosimilar-switch-in-patients-with-inflammatory-rheumatic-diseases-real-world-evidence-from-the-nationwide-danish-registry-danbio/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adalimumab-biosimilar-to-biosimilar-switch-in-patients-with-inflammatory-rheumatic-diseases-real-world-evidence-from-the-nationwide-danish-registry-danbio/