Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Treat-to-target (T2T) strategies are focused on proactive and tailored management of disease activity to minimise damage risk and improve long-term outcomes in SLE across age. T2T outcome definitions in childhood-onset SLE (cSLE) have been adapted from those validated in adult-onset SLE, and include Childhood Lupus Low Disease Activity State (cLLDAS), cSLE clinical remission on-corticosteroids (cCR) and cSLE clinical remission off-corticosteroids (cCR-0).
The feasibility and impact of active implementation of T2T strategies has not been evaluated before in cSLE.
This prospective real-life cSLE quality improvement study aimed to assess the feasibility of agreeing and documenting a treatment target in cSLE, as well as explore the impact of setting cLLDAS as therapeutic target on disease states over 12-month routine follow-up.
Methods: AYA with cSLE and complete disease status data collection at baseline (inclusion over 6 months) and evaluation of disease control against agreed treatment target at least at two time points over a prospective period of 12 months routine follow-up were included. Results are presented using descriptive statistics.
Results: During Oct 2022-April 2024, 135/162 AYA with cSLE have been eligible for inclusion: mean age 26.5±5.1 years, mean disease duration of 13.5 ±4.8 years; 85.1% females, and 31.8% White, 28.1% Black African/Afro-Caribbean, 15.1% Asian and 25% other ethnicity.
Implementing routine outcome measure collection in practice is feasible: only 13/135 (9.8%) AYA with cSLE had incomplete assessments or no therapeutic target discussed/recorded.
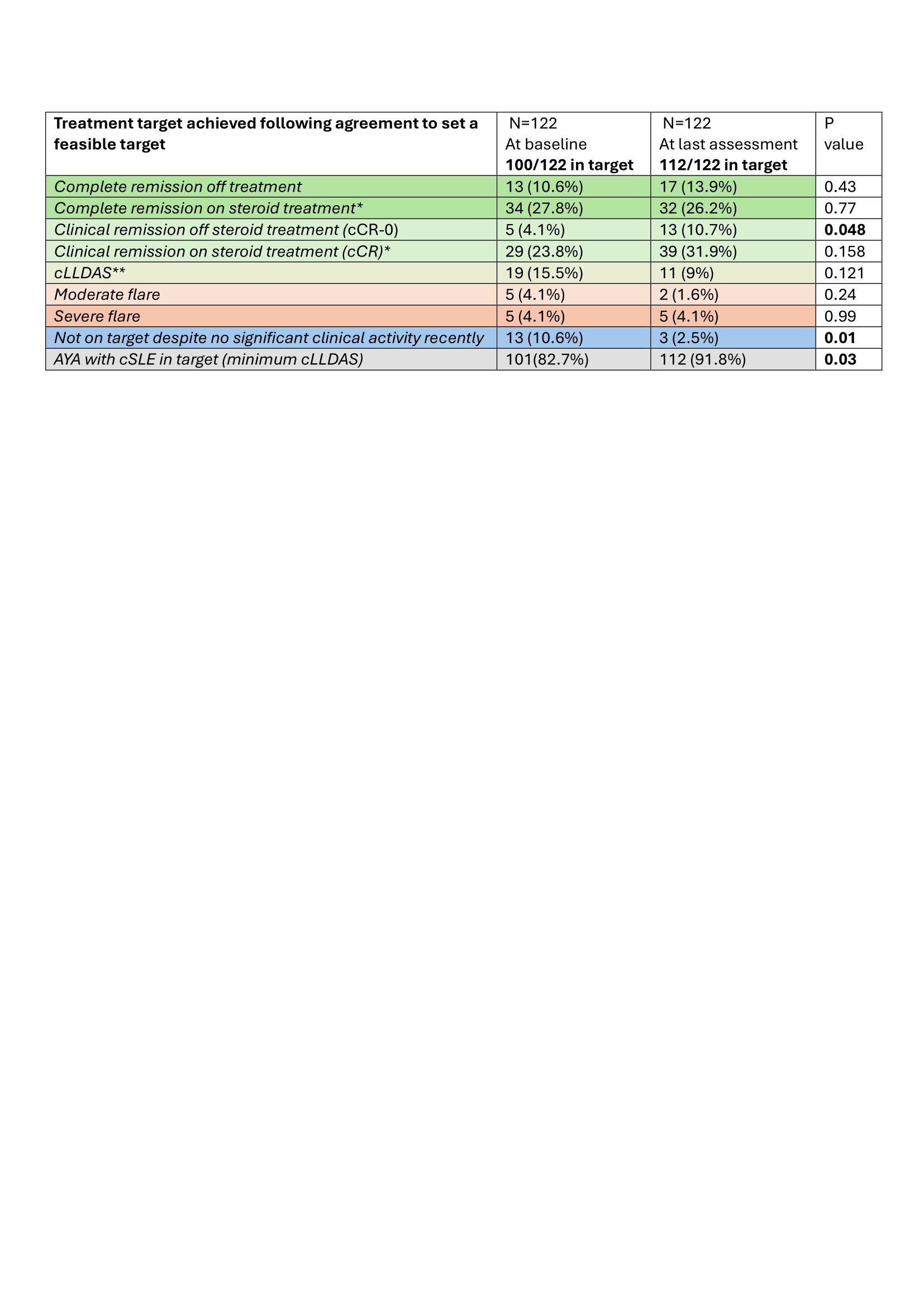
Agreeing with AYA on a treatment target is achievable: 122/135 (90.4%) had a therapeutic target initially agreed and assessed against at least at two, and 82/122 (67.2%) at three time points over 12-months routine follow-up. The disease activity states at the clinical appointment at which the target was agreed (baseline) is detailed in Table 1.
Setting cLLDAS as minimum therapeutic target in cSLE was associated with improved disease control: 14/122 (11.5%) of AYA with cSLE (with active disease or on a higher steroid dose than clinically indicated) achieved the proposed target, while 7/122 (5.7%) previously in target, achieved an even better therapeutic target. After 12 months, a significant improvement in the proportion of AYA achieving cCR-0 (4.1% vs. 10.7, P=0.048) and decrease in the number of AYA not on target (10.6 % vs. 2.5%) have been achieved (Table 1).
Conclusion: Actively implementing a T2T strategy led to an increase in the proportion of AYA achieving minimum cLLDAS as therapeutic target from 82.7% (N=101) to 91.8% (N=112) over a 12-month period (P=0.03), suggesting that clinician/patient education and co-operation could improve cSLE disease control. This study provides the much needed evidence that T2T strategies are an achievable goal in AYA with cSLE in routine practice.
* Equivalent of prednisolone ≤0.1 mg/kg daily, maximum of 5 mg daily;
** Equivalent of prednisolone ≤0.15 mg/kg daily, maximum of 7.5 mg daily;
To cite this abstract in AMA style:
Gotch R, Ahmed Y, Wilson R, Hawkins E, Ciurtin C. Active Implementation of Low Disease Activity State as Treatment Endpoint in a Large Cohort of Adolescents and Young Adults with Childhood Onset Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/active-implementation-of-low-disease-activity-state-as-treatment-endpoint-in-a-large-cohort-of-adolescents-and-young-adults-with-childhood-onset-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/active-implementation-of-low-disease-activity-state-as-treatment-endpoint-in-a-large-cohort-of-adolescents-and-young-adults-with-childhood-onset-systemic-lupus-erythematosus/