Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Bimekizumab (BKZ) is a monoclonal antibody that selectively inhibits interleukin (IL)-17F in addition to IL-17A. BKZ has demonstrated sustained efficacy and safety to Week (Wk) 52 in patients (pts) across the full spectrum of axial spondyloarthritis (axSpA) in the phase 3 studies BE MOBILE 1 (non-radiographic [nr-]axSpA) and 2 (radiographic [r-]axSpA).1
Achievement of remission is a treatment goal and may guide clinical decisions.2,3 Here, we report achievement of remission defined using objective signs of inflammation (OSI) in pts with axSpA compared with an established measure of remission, Axial Spondyloarthritis Disease Activity Score < 1.3 (ASDAS Inactive Disease [ID]).
Methods: BE MOBILE 1 (NCT03928704) and 2 (NCT03928743) comprised a 16-wk double-blind, placebo (PBO) controlled period followed by a 36-wk maintenance period. Pts were randomized to subcutaneous BKZ 160 mg every 4 wks (Q4W) or PBO, with all pts receiving BKZ from Wk 16 onwards.
Remission of OSI comprised MRI remission of the sacroiliac joints (SIJ) and spine (MRI Spondyloarthritis Research Consortium of Canada [SPARCC] SIJ score < 2 and Berlin MRI spine ≤2), C-reactive protein (CRP) ≤5 mg/L and a swollen joint count (SJC) of 0. The proportion of pts from BE MOBILE 1 and 2 MRI sub-studies achieving these criteria was compared with those achieving ASDAS ID using observed case (OC) data.
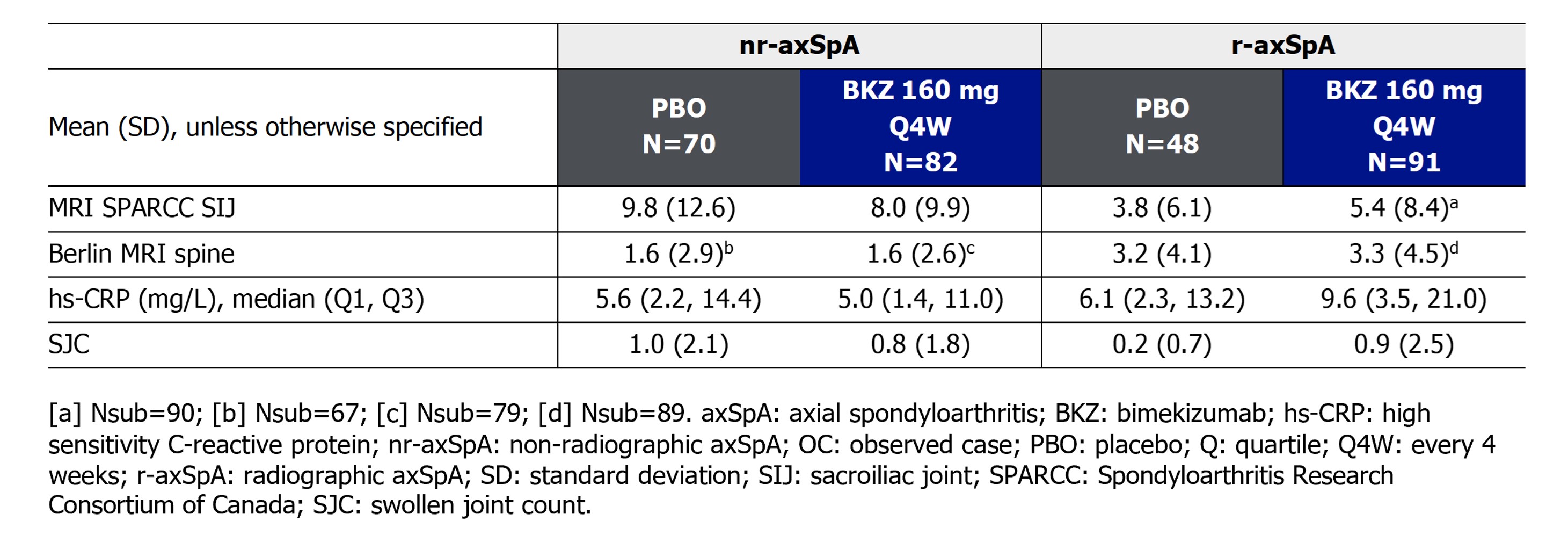
Results: 152 and 139 pts from the MRI sub-studies of BE MOBILE 1 and 2, respectively, were included in this analysis. Levels of objective signs of inflammation at baseline were similar across treatment arms in pts with nr-axSpA and r-axSpA, respectively (Table).
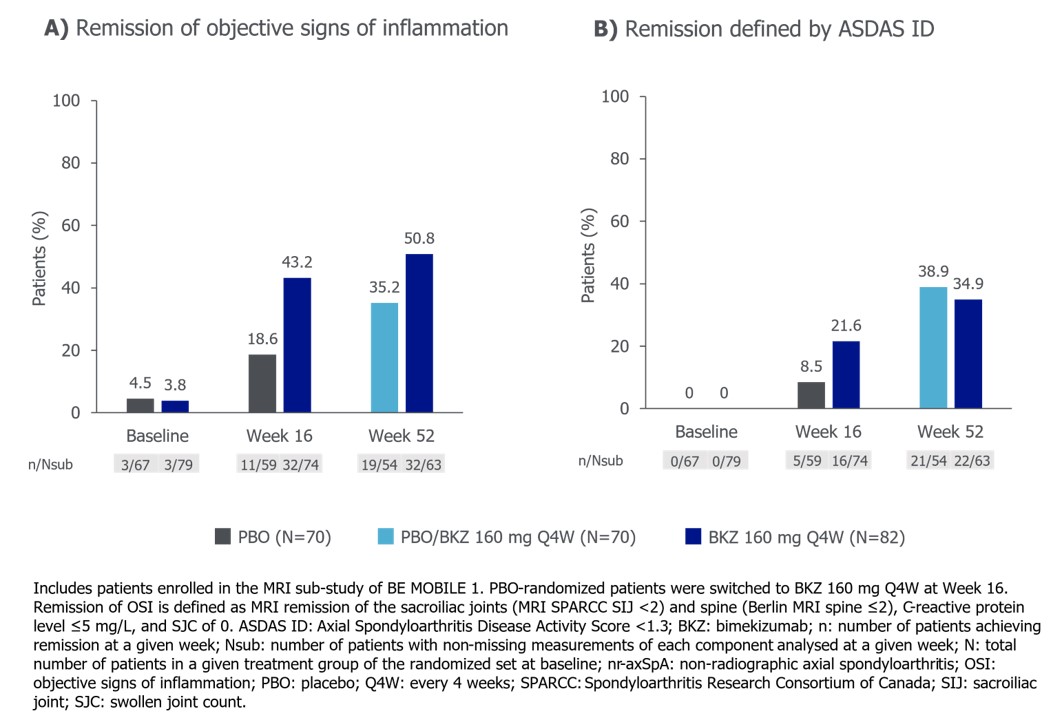
Among pts with nr-axSpA, 32/74 (43.2%) BKZ-randomized pts achieved remission of OSI at Wk 16, compared with 16/74 (21.6%) achieving ASDAS ID. 11/59 (18.6%) PBO-randomized pts achieved remission of OSI at Wk 16, compared with 5/59 (8.5%) achieving ASDAS ID. 32/63 (50.8%) BKZ-randomized pts achieved remission of OSI at Wk 52, compared with 22/63 (34.9%) achieving ASDAS ID. Having switched to BKZ at Wk 16, 19/54 (35.2%) PBO-randomized patients achieved remission of OSI at Wk 52, compared with 21/54 (38.9%) achieving ASDAS ID (Figure 1).
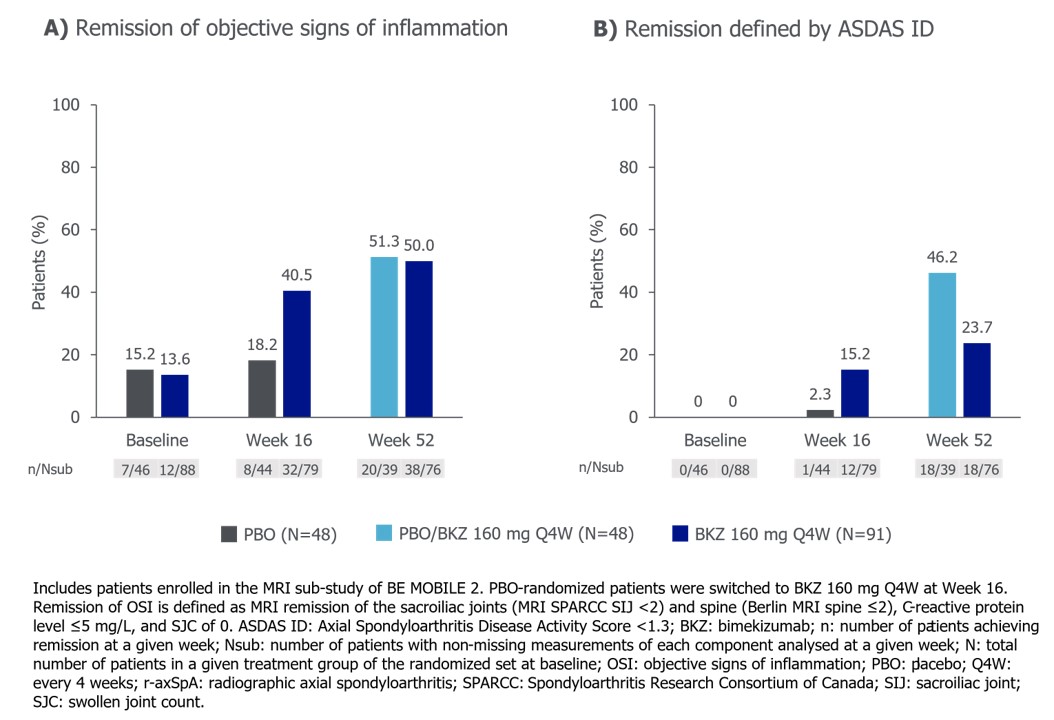
In pts with r-axSpA, 32/79 (40.5%) BKZ-randomized pts achieved remission of OSI at Wk 16, compared with 12/79 (15.2%) achieving ASDAS ID. Among PBO-randomized patients, 8/44 (18.2%) achieved remission of OSI at Wk 16, compared with 1/44 (2.3%) achieving ASDAS ID. 38/76 (50.0%) BKZ-randomized patients achieved remission of OSI at Wk 52, compared with 18/76 (23.7%) achieving ASDAS ID. Having switched to BKZ at Wk 16, 20/39 (51.3%) PBO-randomized patients achieved remission of OSI at Wk 52, compared with 18/39 (46.2%) achieving ASDAS ID (Figure 2).
Conclusion: In general, a higher proportion of patients treated with BKZ achieved remission defined by the absence of OSI compared to ASDAS ID. These findings suggest that ASDAS ID could underestimate the anti-inflammatory effects of BKZ, while also highlighting a potential need for optimized endpoints to guide clinical treatment management in axSpA.
References: 1. Baraliakos X. Ann Rheum Dis 2024;83:199–213; 2. Smolen JS. Ann Rheum Dis 2018;77:3–17; 3. Ramiro S. Ann Rheum Dis 2023;82:19–34.
To cite this abstract in AMA style:
Gensler L, Marzo-Ortega H, Taieb V, Voiniciuc D, Marten A, Stojan G, Kim M, Rudwaleit M. Achievement of Remission Defined by Absence of Objective Signs of Inflammation versus ASDAS ID in Patients with Active Axial Spondyloarthritis Treated with Bimekizumab: 52-Week Results from Two Phase 3 Studies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/achievement-of-remission-defined-by-absence-of-objective-signs-of-inflammation-versus-asdas-id-in-patients-with-active-axial-spondyloarthritis-treated-with-bimekizumab-52-week-results-from-two-phase/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/achievement-of-remission-defined-by-absence-of-objective-signs-of-inflammation-versus-asdas-id-in-patients-with-active-axial-spondyloarthritis-treated-with-bimekizumab-52-week-results-from-two-phase/