Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Painful and recurring oral ulcers (OU) are a common manifestation of Behçet’s syndrome that can interfere with eating and have a negative impact on quality of life (Ozguler Y, et al. Clin Exp Rheumatol. 2019;37[6 Suppl 121]:28‐34). Apremilast (APR), an oral phosphodiesterase 4 inhibitor, has demonstrated efficacy in the treatment of OU in the phase 3 RELIEF study (Hatemi G, et al. N Engl J Med. 2019;381:1918-1928). In RELIEF, 53% of patients treated with APR 30 mg BID and 22% who received placebo (PBO) achieved OU complete response at Week 12 (Hatemi G, et al. N Engl J Med. 2019;381:1918-1928). Here, we describe the achievement of OU complete response over different time points during the PBO-controlled period in RELIEF.
Methods: This multicenter, randomized, double-blind, PBO-controlled study (BCT-002; NCT02307513) enrolled adult patients (≥18 years of age) with active Behçet’s syndrome and ≥3 OU at randomization or ≥2 OU at screening and randomization, without active major organ involvement. Patients were randomized (1:1) to receive APR or PBO during the 12-week PBO-controlled period. The current post hoc analysis assessed the proportion of individual patients who achieved OU complete response (ie, were OU-free) at any point during the PBO-controlled period and the proportion of patients who achieved complete response for OU as early as Weeks 1 or 2. The number of patients achieving OU complete response by Week 6 who remained OU-free for ≥6 additional weeks was also evaluated. Data are summarized descriptively.
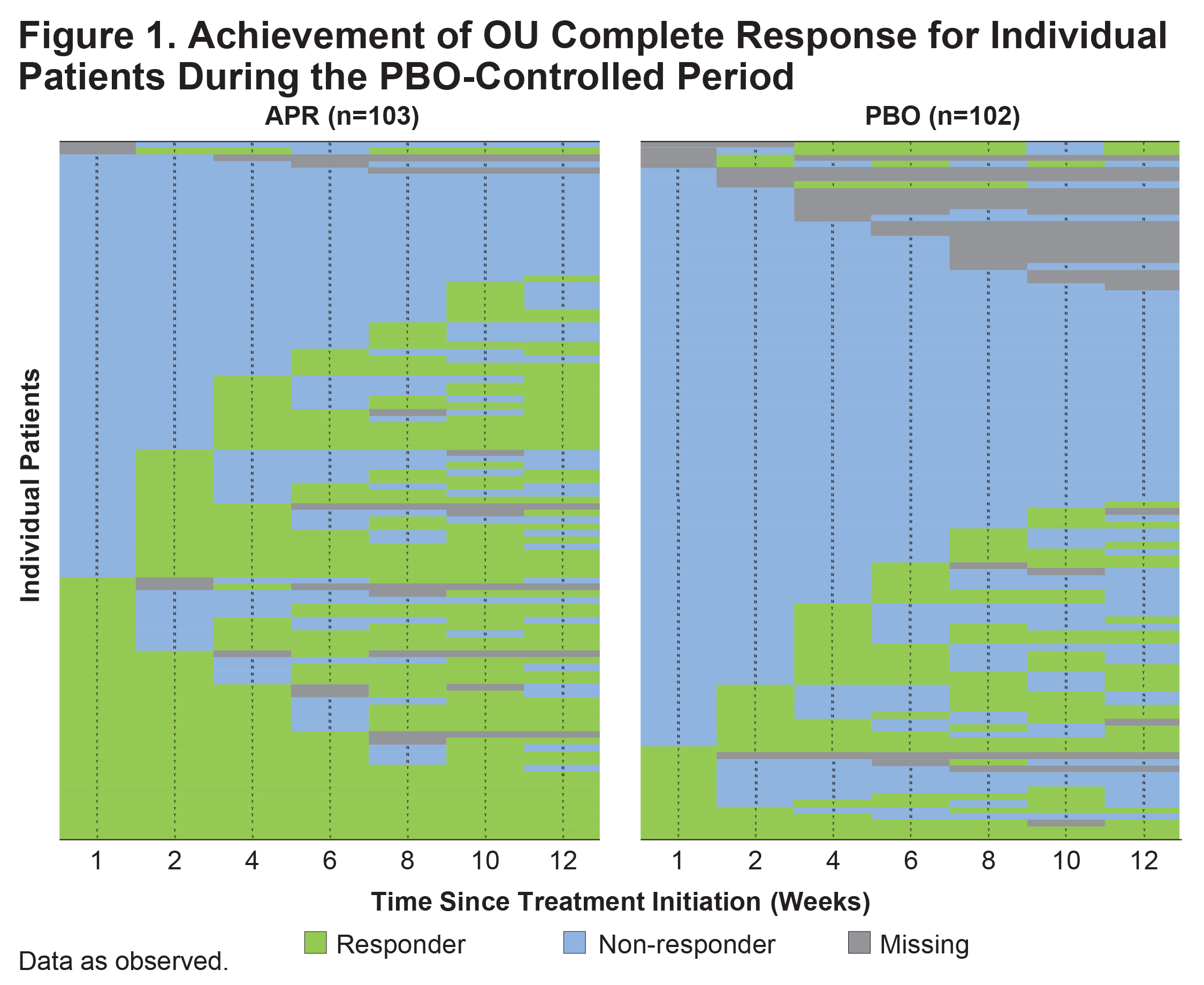
Results: A total of 207 patients were randomized and received ≥1 dose of study medication, including 104 who received APR and 103 who received PBO. At baseline, the mean (SD) number of OU was 4.2 (3.7) in the APR group and 3.9 (2.7) in the PBO group. Mean (SD) duration of Behçet’s syndrome at baseline was 6.7 (7.4) years in the APR group and 6.9 (8.0) years in the PBO group. The proportion of individual patients who achieved OU complete response at any time point during the 12-week PBO-controlled period was higher with APR than with PBO (Figure 1). Greater proportions of patients achieved early OU complete response during Weeks 1 or 2 with APR compared with PBO (Figure 2). In total, 44/104 patients receiving APR and 24/103 patients receiving PBO had OU complete response by Week 6; of these, 33 patients in the APR group and 12 in the PBO group maintained OU-free response for ≥6 additional weeks.
Conclusion: In the RELIEF study, a higher proportion of patients with active Behçet’s syndrome achieved OU complete response with APR treatment compared with PBO during the PBO-controlled period. Similarly, a greater proportion of patients achieved early OU complete response and maintained OU-free response with APR compared with PBO.
To cite this abstract in AMA style:
Hatemi G, Mahr A, Takeno M, Kim D, Melikoğlu M, Cheng S, Richter S, Brunori M, Paris M, Chen M, Yazici Y. Achievement of Early and Sustained Complete Response of Oral Ulcers with Apremilast Compared with Placebo in Patients with Active Behçet’s Syndrome [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/achievement-of-early-and-sustained-complete-response-of-oral-ulcers-with-apremilast-compared-with-placebo-in-patients-with-active-behcets-syndrome/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/achievement-of-early-and-sustained-complete-response-of-oral-ulcers-with-apremilast-compared-with-placebo-in-patients-with-active-behcets-syndrome/