Session Information
Date: Sunday, November 13, 2022
Title: Abstracts: Spondyloarthritis Including PsA – Treatment II: PsA
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Psoriatic arthritis (PsA) is a chronic, inflammatory disease with multiple clinical manifestations (arthritis, spondylitis, enthesitis, dactylitis, skin, nails). IL-17 inhibitors have shown efficacy across all disease domains. Izokibep, a first-in-class fusion protein, is a unique IL-17A inhibitor with very high potency (Kd =0.3 pM), small molecular size (18.6 kD versus mAbs ~ 150 kD), and attachment to albumin. Treat to target is an important strategy to optimize disease control (reducing inflammation, limiting damage) as well as optimizing quality of life in PsA. Many composite measures of disease control have been proposed for clinical decision-making, including ACR scores, DAS28-CRP, DAPSA scores, and MDA. Here, we report the efficacy results of izokibep 40 mg Q2W and 80 mg Q2W versus placebo across a range of disease outcome measures as well as relevant safety data over 16 weeks compared to placebo.
Methods: A multicenter, randomized, double-blind, placebo-controlled, study of placebo, izokibep 40 mg Q2W or 80 mg Q2W delivered subcutaneously to Week 16 (ClinicalTrials.gov, NCT04713072). PsA patients met CASPAR criteria and required ≥3 swollen and ≥3 tender joints on the 66/68 joint count, an inadequate response to previous NSAIDs, csDMARDs, or TNF inhibitors to enroll. ACR50 was the primary endpoint for izokibep 80 mg Q2W versus placebo at Week 16. Other measures of disease response included ACR20/70, DAS28-CRP, DAPSA, MDA, and PASI scores. Here we report achievement of disease activity responses over time on DAS-28CRP and DAPSA and of efficacy thresholds for MDA and PASI 75, 90, and 100.
Safety and tolerability were assessed.
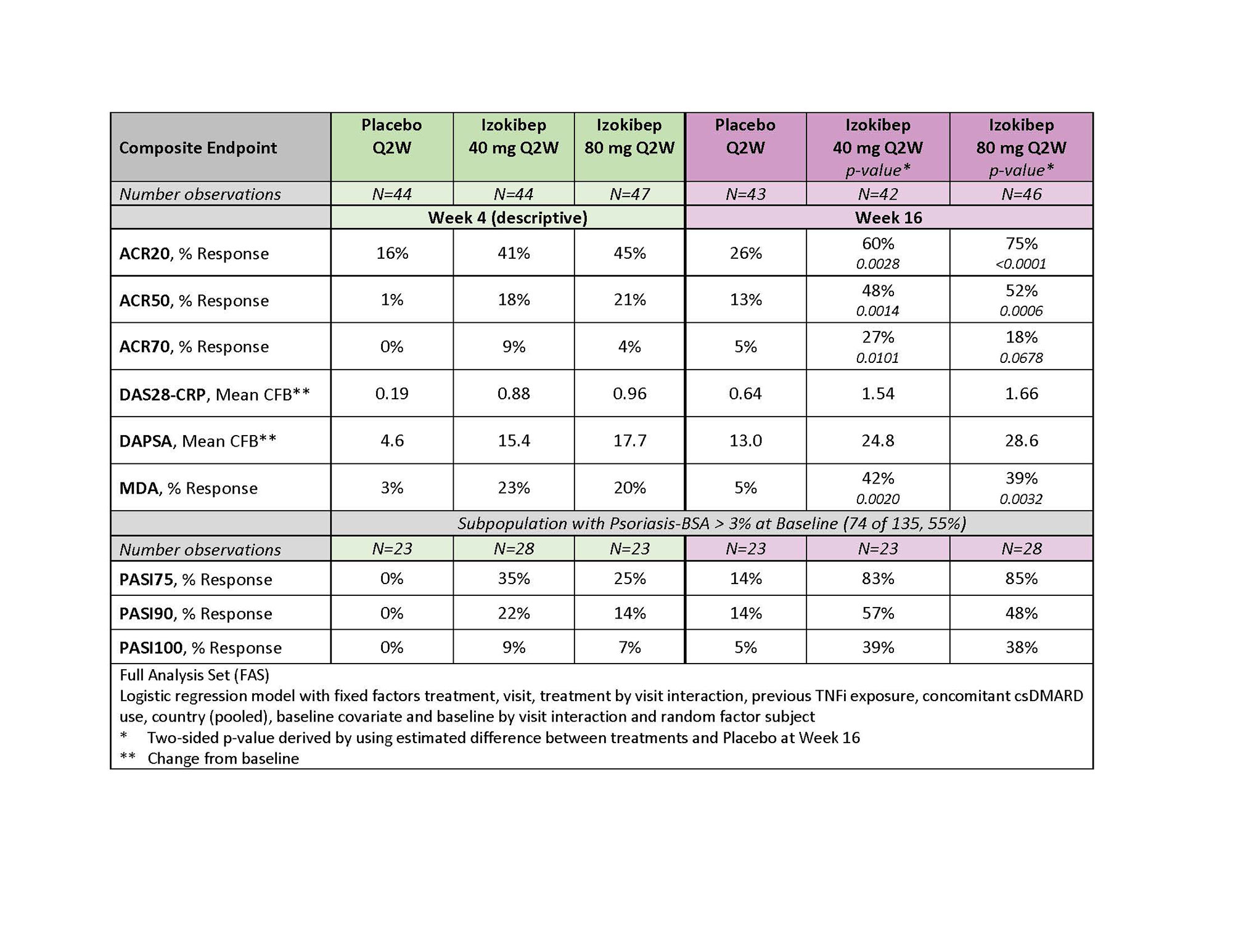
Results: 135 patients were randomized at 28 sites in 7 EU countries between June 2020 and July 2021. At randomization, patients had a mean age of 48.5 years, mean BMI of 29.0 kg/m2, mean swollen joint count of 9.9, mean tender joint count of 16.7, and mean PsA duration of 7.1 years. 80% were receiving a concomitant csDMARD and 13% had failed a TNF inhibitor. At baseline, the mean DAS28-CRP was 4.51 and the mean baseline DAPSA score was 46.8. At Week 16, the primary endpoint ACR50 was met in 13% for placebo, 48% for the 40 mg Q2W group (p=0.0014), and 52% for the 80 mg Q2W group (p=0.0006). Minimal disease activity (MDA) was achieved in 42% (40mg; p=0.0020), 39% (80mg; 0.0032) versus 5% (Placebo) at Week 16 (Figure 1).
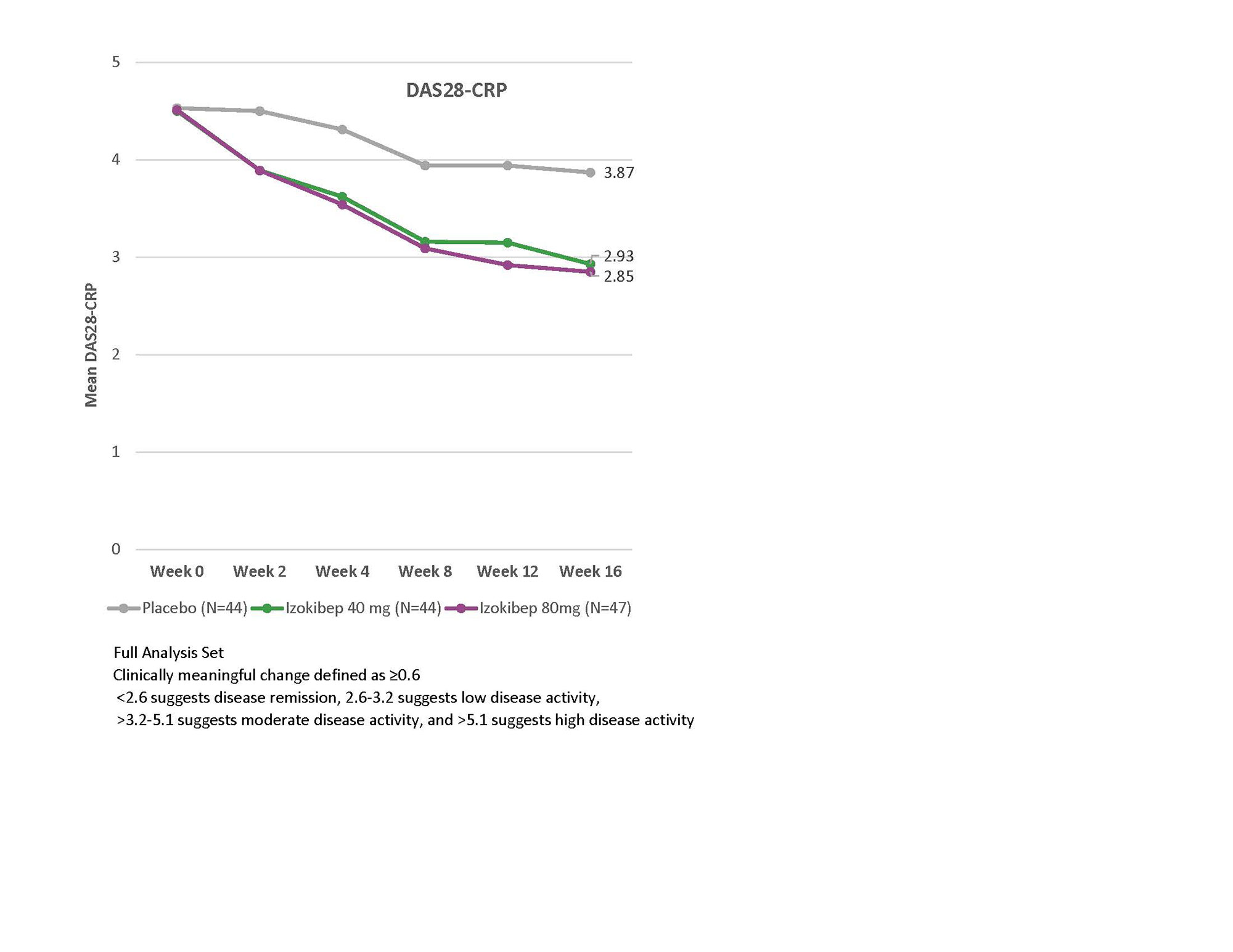
Table 1 shows treatment effects for other endpoints at Week 4 and 16. The course of the DAS28-CRP shows early response and separation from placebo, a clinically meaningful change (≥0.6) between izokibep treatment groups and placebo (Figure 2) as early as 2 weeks. Eighty-three % (40 mg), and 85% (80 mg) of patients with a PSO-BSA > 3% (74 of 135, 55%) achieved a PASI75 response versus 14% (Placebo) at Week 16.
Adverse event rates for izokibep were mostly similar to placebo and consistent with the IL-17A inhibitors currently available.
Conclusion: Izokibep showed consistently high levels of response across multiple disease measures of joint and skin improvement, beginning early and with ongoing improvement. The safety profile of izokibep was generally consistent with other IL-17A inhibitors, and in this trial, event rates were mostly similar to placebo.
To cite this abstract in AMA style:
Behrens F, Taylor P, Mease P, Peloso P, Wetzel D, Brun N, Wiens B, Brandt-Juergens J, Drescher E, Dokoupilova E, Rowińska-Osuch A, Abdel- Kader Martin N, de Vlam K. Achievement of Different Treatment Targets with Izokibep Demonstrates Efficacy Benefits in Patients with Active Psoriatic Arthritis: Results from a 16-Week Randomized, Placebo-Controlled Phase 2 Clinical Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/achievement-of-different-treatment-targets-with-izokibep-demonstrates-efficacy-benefits-in-patients-with-active-psoriatic-arthritis-results-from-a-16-week-randomized-placebo-controlled-phase-2-clini/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/achievement-of-different-treatment-targets-with-izokibep-demonstrates-efficacy-benefits-in-patients-with-active-psoriatic-arthritis-results-from-a-16-week-randomized-placebo-controlled-phase-2-clini/