Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Glucocorticoids (GCs) are used commonly to treat patients with rheumatoid arthritis (RA) and other inflammatory conditions. As clinical trials are often underpowered to assess safety, administrative claims data has been used to study GC safety. This study aimed to validate the use of claims prescription fill data for identifying current GC use and dose, and to assess how GC exposure misclassification affects estimates of GC risk.
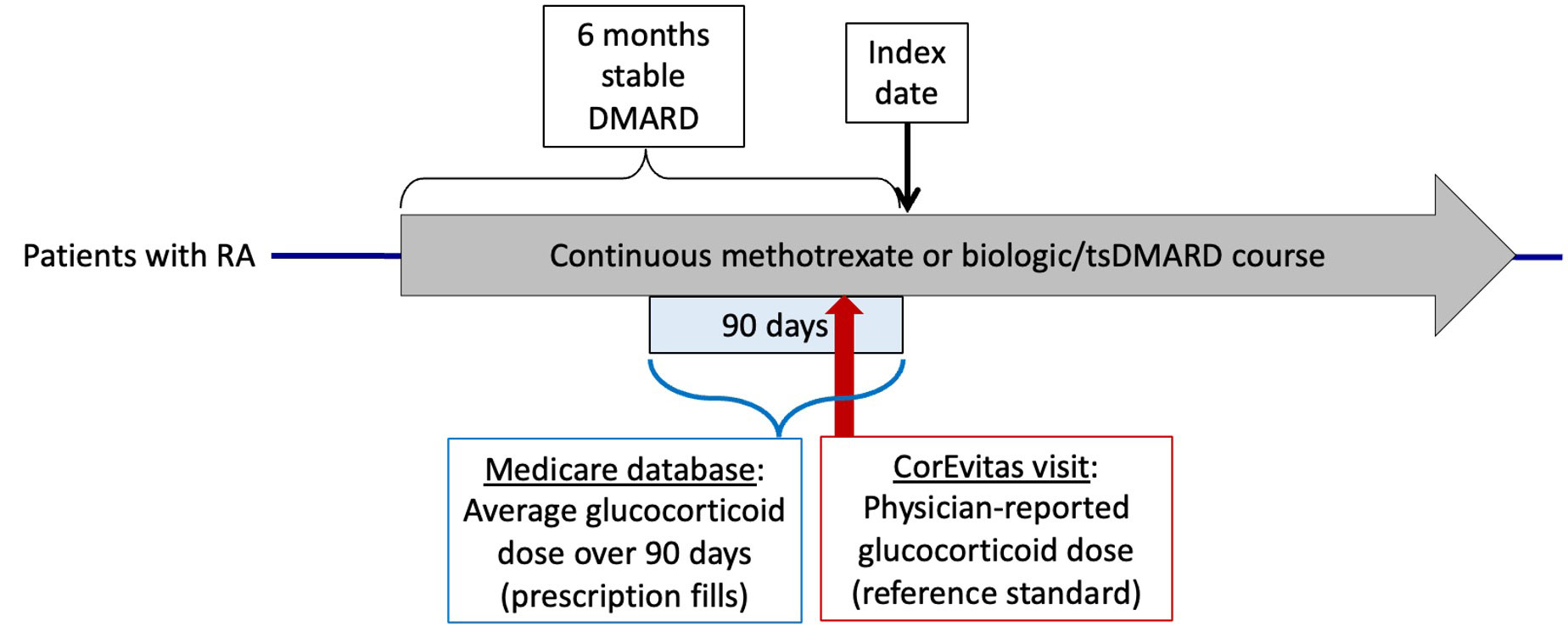
Methods: Using Medicare claims data from 2006-2015, we identified adults with RA who were on stable DMARD therapy for 6 months and had linked data available in the CorEvitas registry. The index date was defined as 6 months after patients started a stable DMARD course. Medicare prescription fill data was used to estimate current GC use (yes/no) and dose, averaging prescriptions in the 90 days prior to the index date as in prior studies to account for uncertainty in prescription durations. These estimates were compared to rheumatologist-reported GC use and dose from a CorEvitas visit (reference standard) during the same 90-day period (Figure 1), using weighted kappa to compare dose categories (none, ≤5mg, 5-10mg, >10mg/day). Sensitivity analyses examined different claims algorithms to estimate dose (e.g. alternative handling of overlapping or duplicate prescriptions), alternative dose categories (0-2mg, ≥2 to ≤ 5mg, 5-10mg, >10mg/day) to account for brief GC prescriptions unlikely to be captured in registry data, and an analysis was performed excluding patients with dose changes at the CorEvitas visit. Lastly, we used the sensitivity and specificity for GC use to conduct a deterministic sensitivity analysis with the Stata episensi package, evaluating the effect of GC exposure misclassification on estimates of infection risk with GCs from a prior Medicare study.
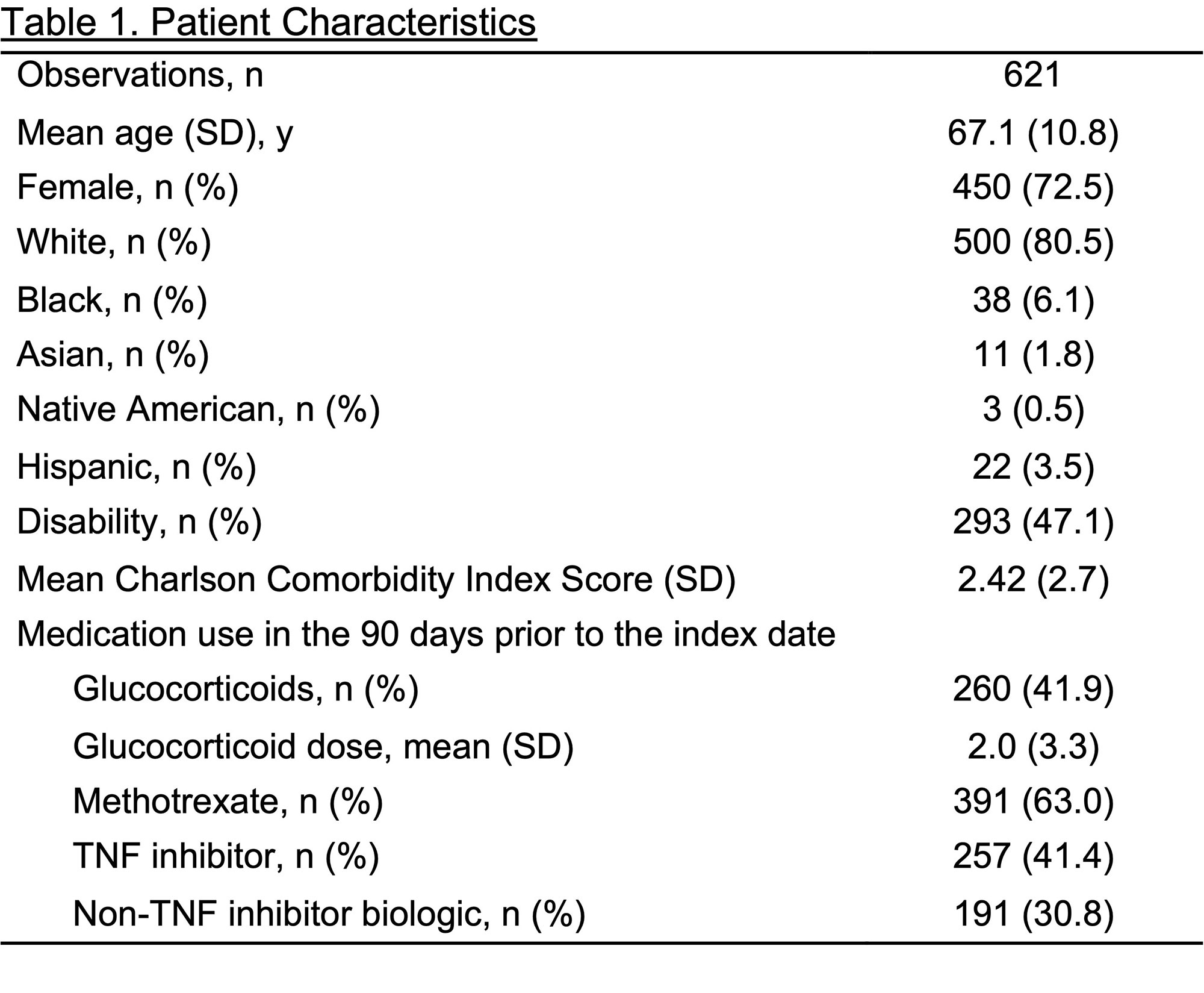
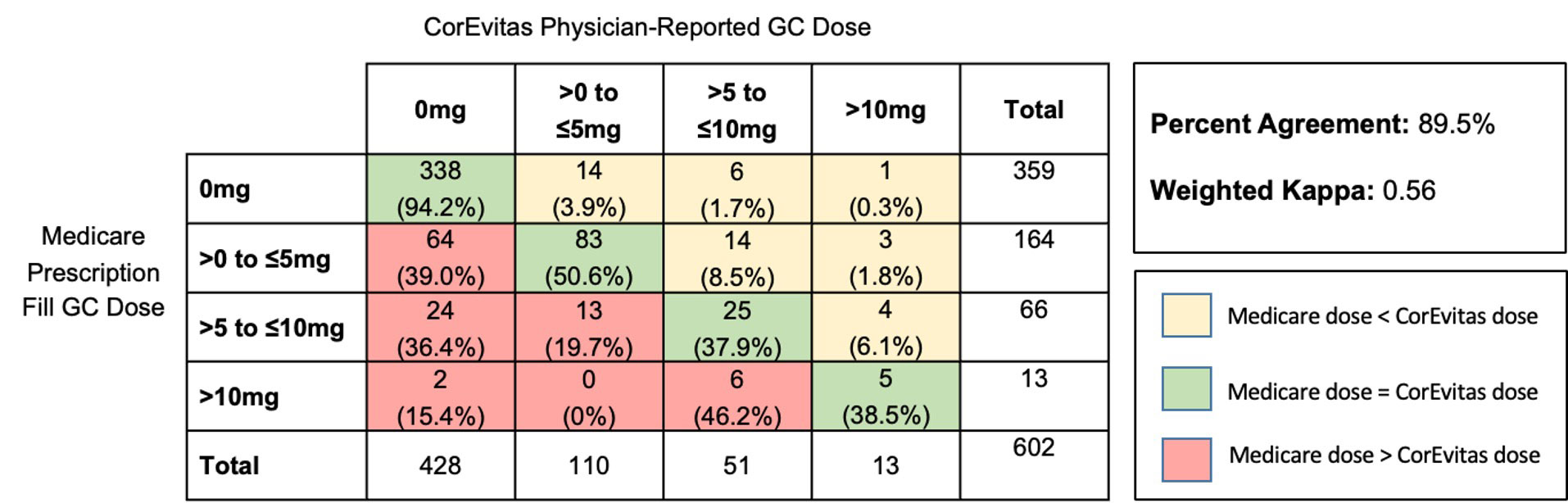
Results: We identified 621 unique stable DMARD course observations among 494 patients with linked data. Patients had mean (SD) age 67 (11) and 73% were female (Table 1). We identified GC use in 41.9% of observations using prescription fill data versus 31.1% by CorEvitas physician-report. For any GC use, prescription fill data had sensitivity 88.1% (95% CI 82.7-92.3), specificity 79.0% (74.8-82.7), PPV 65.4% (59.3-71.2), NPV 93.6% (90.6-95.9), and 81.8% agreement with CorEvitas, with kappa 0.61 (substantial agreement). For GC dose, there was 89.5% agreement between prescription fill and physician-reported dose categories with weighted kappa 0.56 (moderate agreement), with prescription fill tending to overestimate GC dose (Figure 2). Similar results were obtained in sensitivity analyses. Applying the sensitivity and specificity for GC use to a previous Medicare study examining infection risk with GCs, we found that the estimated risk ratio of 1.44 (95% CI 1.41-1.48) in that study would be adjusted to 1.74 after accounting for exposure misclassification.
Conclusion: Claims prescription fill data can be used to estimate GC use and dose, particularly in patients with RA. Claims data may overestimate GC use and dose, however, leading to underestimates of GC risks. Our results can be applied to externally adjust risk estimates in other studies that use prescription fill data to estimate GC use.
To cite this abstract in AMA style:
Galvao R, Curtis J, Harrold L, Wu Q, Xie F, George M. Accuracy of Administrative Claims Prescription Fill Data to Estimate Glucocorticoid Use and Dose in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/accuracy-of-administrative-claims-prescription-fill-data-to-estimate-glucocorticoid-use-and-dose-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/accuracy-of-administrative-claims-prescription-fill-data-to-estimate-glucocorticoid-use-and-dose-in-patients-with-rheumatoid-arthritis/