Session Information
Session Type: ACR Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose:
Many
patients fail to reach target serum urate (SU) on allopurinol. Although the most
common causes of inadequate response are non-adherence and low dosing in kidney
disease, there are a group of adherent patients who fail to achieve target SU and
can be considered poor responders to allopurinol. In patients who have
undergone allopurinol dose escalation, poor response can be defined as failure
to reach the recommended target SU of <6mg/dl on allopurinol ≤300mg/day.
A recent GWAS identified the urate raising minor allele of ABCG2 rs2231142
as a determinant of poor allopurinol response [1]. However, the definition
of response used in the GWAS was allopurinol related SU change with only weak assessment
of adherence. The aim of our study was to examine the association of ABCG2
rs2231142 with allopurinol response in a well phenotyped group of
patients with gout.
Methods: Patients with gout
according to 1977 ARA criteria participating in clinical trials of allopurinol
were recruited. Good response was defined as SU <6mg/dl on
allopurinol ≤300mg/d and poor response was defined as SU ≥6mg/dl
despite allopurinol >300mg/d. Adherence was assessed by plasma oxypurinol
concentrations >20umol/l and in the absence of oxypurinol concentration SU
reduction with allopurinol dose escalation. Genotyping for ABCG2 (rs2231142)
was performed using a pre-designed SNP TaqMan assay. Logistic regression
analyses were used to test for an association between poor response to
allopurinol and rs2231142. Adjustments were made for age, sex, body
mass index (BMI) and ethnicity and further separate adjustments were made for eGFR,
diuretic use and SU off urate lowering therapy.
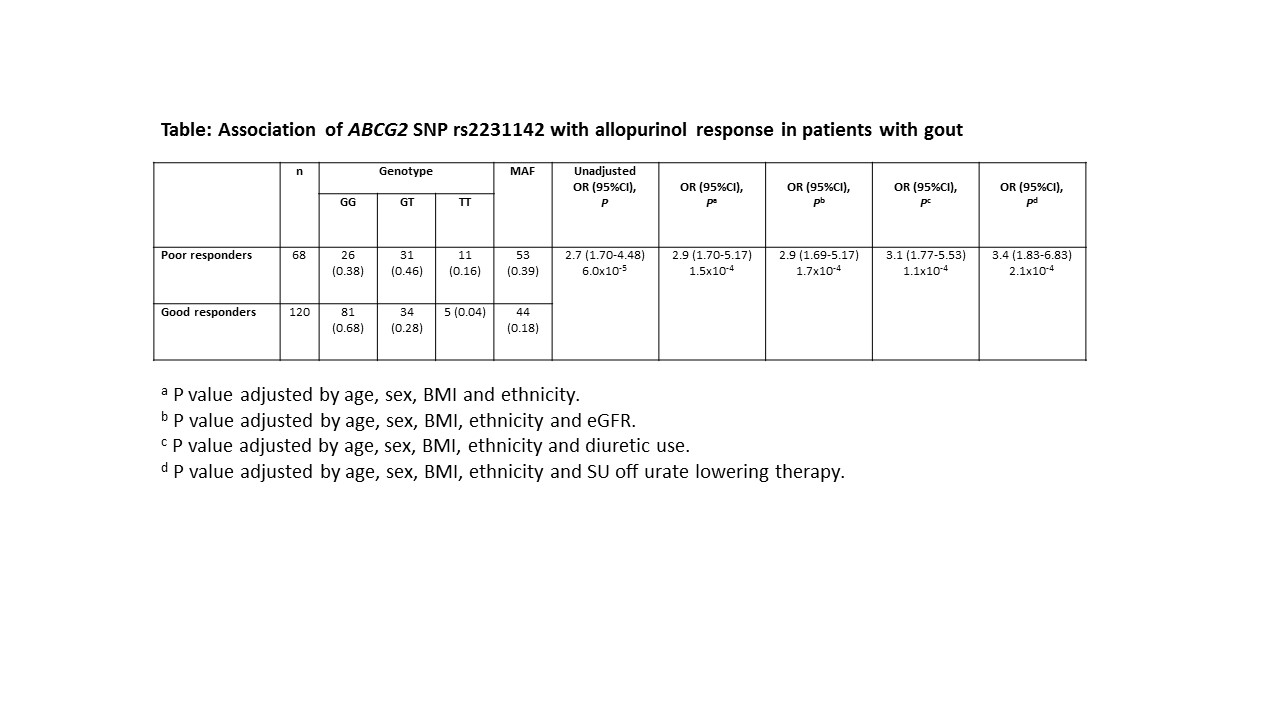
Results : Of 264 patients with
gout receiving allopurinol, 120 (45.4%) patients were good responders, 68
(25.8%) were poor responders and 76 (28.8%) were either non-adherent or could
not be classified. Of the 188 patients included in the responder analysis, 85.6%
were male, mean age was 59 years and 32% were receiving a diuretic. The mean
BMI was 34.1 kg/m2 and eGFR 55.5mls/min/1.72m2. Mean SU
prior to urate lowering therapy was 10.1 mg/dl. Mean SU was 5.2 mg/dl in good
responders and 7.0mg/dl in poor responders. Mean allopurinol dose was 263 mg/d
(100-300) in the good responders and 413 mg/d (350-700) in poor responders. Logistic
regression found that the minor allele of ABCG2 rs2231142 was
significantly associated with poor response to allopurinol (Table; OR=2.9, P=1.5×10-4).
This association persisted after adjusting for eGFR, diuretic use and SU off
ULT (Table).
Conclusion: This study demonstrates
that ABCG2 rs2231142 predicts
poor response to allopurinol, as defined by SU ≥6mg/dl despite
allopurinol >300mg/d. ABCG2 genotyping may allow identification of
people with gout for whom standard doses of allopurinol are unlikely to lead to
therapeutic target serum urate levels.
1. Wen C, et al Clin
Pharm Ther 2015, 97(5):518-525.
To cite this abstract in AMA style:
Stamp LK, Wallace M, Merriman TR, Phipps-Green A, Topless R, Drake J, Tan P, Dalbeth N, Roberts R. ABCG2 rs2231142 predicts Poor Response to Allopurinol in Patients with Gout [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/abcg2-rs2231142-predicts-poor-response-to-allopurinol-in-patients-with-gout/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/abcg2-rs2231142-predicts-poor-response-to-allopurinol-in-patients-with-gout/