Session Information
The 2020 Pediatric Rheumatology Symposium, originally scheduled for April 29 – May 2, was postponed due to COVID-19; therefore, abstracts were not presented as scheduled.
Session Type: Poster Breakout Session
Session Time: 5:10PM-5:40PM
Background/Purpose: Juvenile localized scleroderma (jLS) is an autoimmune disease commonly associated with damage. Damage includes dyspigmentation, tissue atrophy, arthropathy, hemiatrophy, vision loss, and seizures. To limit damage severity, methotrexate (MTX), with or without glucocorticoids (GC), is recommended to treat patients with active disease. This regimen is effective in ~70% of patients. Evidence on how to treat non-responders is limited, with some patients having persistently active disease for decades.
Many studies have identified T cells and their associated cytokines in patients with active disease, which suggests abatacept (ABA), a selective T cell co-stimulatory signal inhibitor, could be an effective treatment. A good response to ABA has been reported in small case series of adult onset patients who failed MTX. We report our experience using ABA to treat refractory jLS patients.
Methods: This was a multi-center retrospective cohort study of jLS patients that initiated ABA treatment prior to 7/1/18. Inclusion criteria included diagnosis of jLS, with onset < 16 years. Charts were reviewed to extract demographics, disease characteristics, medication history, and reasons for ABA use. Data was collected at start of ABA (baseline), and at 6 month intervals up to 24 months, or ABA discontinuation, whichever was sooner. Descriptive statistics analysis was performed.
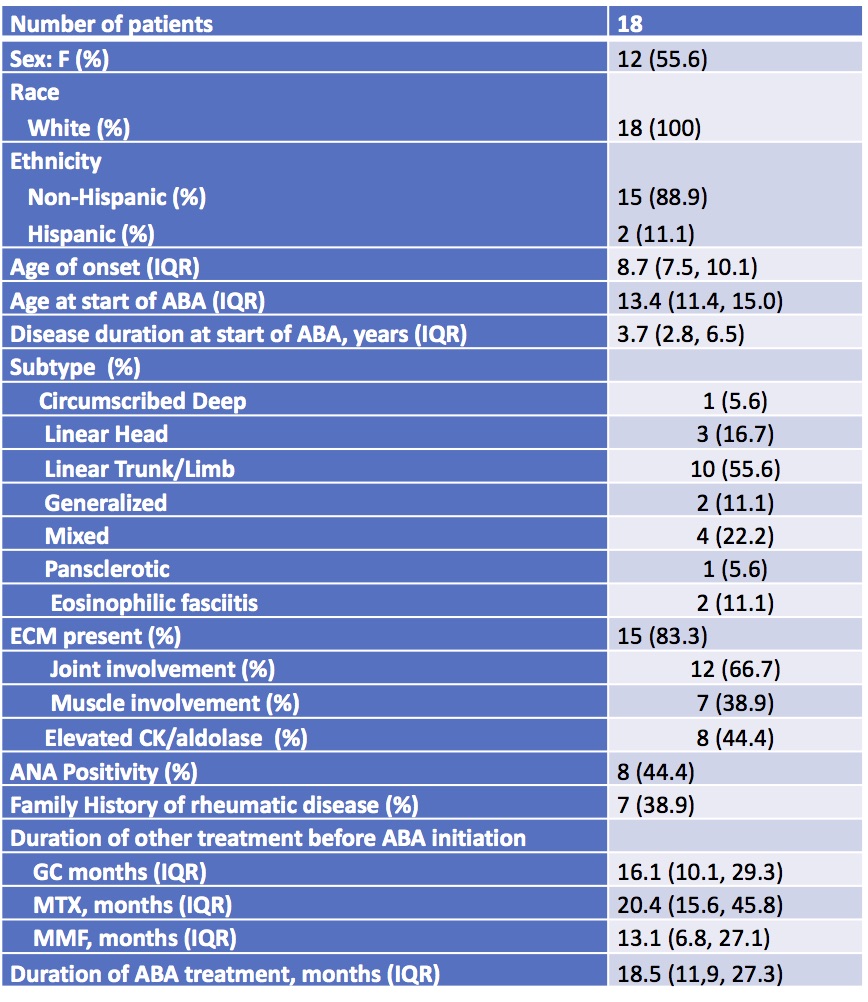
Results: 18 patients were evaluated, median age 13.4 years, disease duration 3.7 years. Most had linear scleroderma (Table 1). Prior treatment included MTX (17), mycophenolate mofetil (MMF, 16), and GC (17), each with median treatment duration >1 year (Table 1). Reasons for ABA use included persistent disease or flare (14, 78%), intolerance of other medications (5, 28%), and GC dependence (4, 22%). ABA was dosed per JIA recommendations, with 16 treated intravenously. All patients concurrently received other treatment: MTX (12), MMF (10), hydroxychloroquine (1), and GC (14).
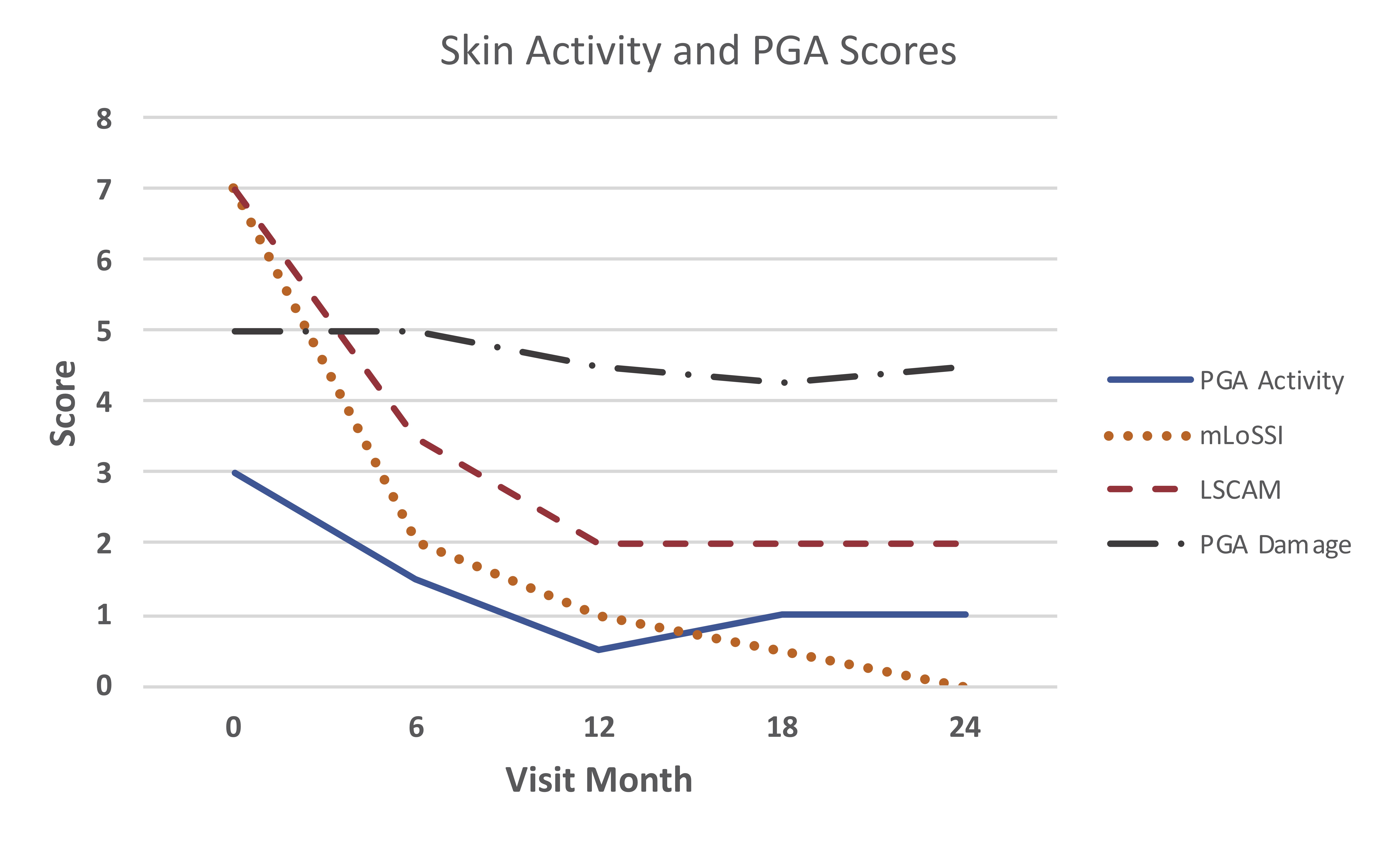
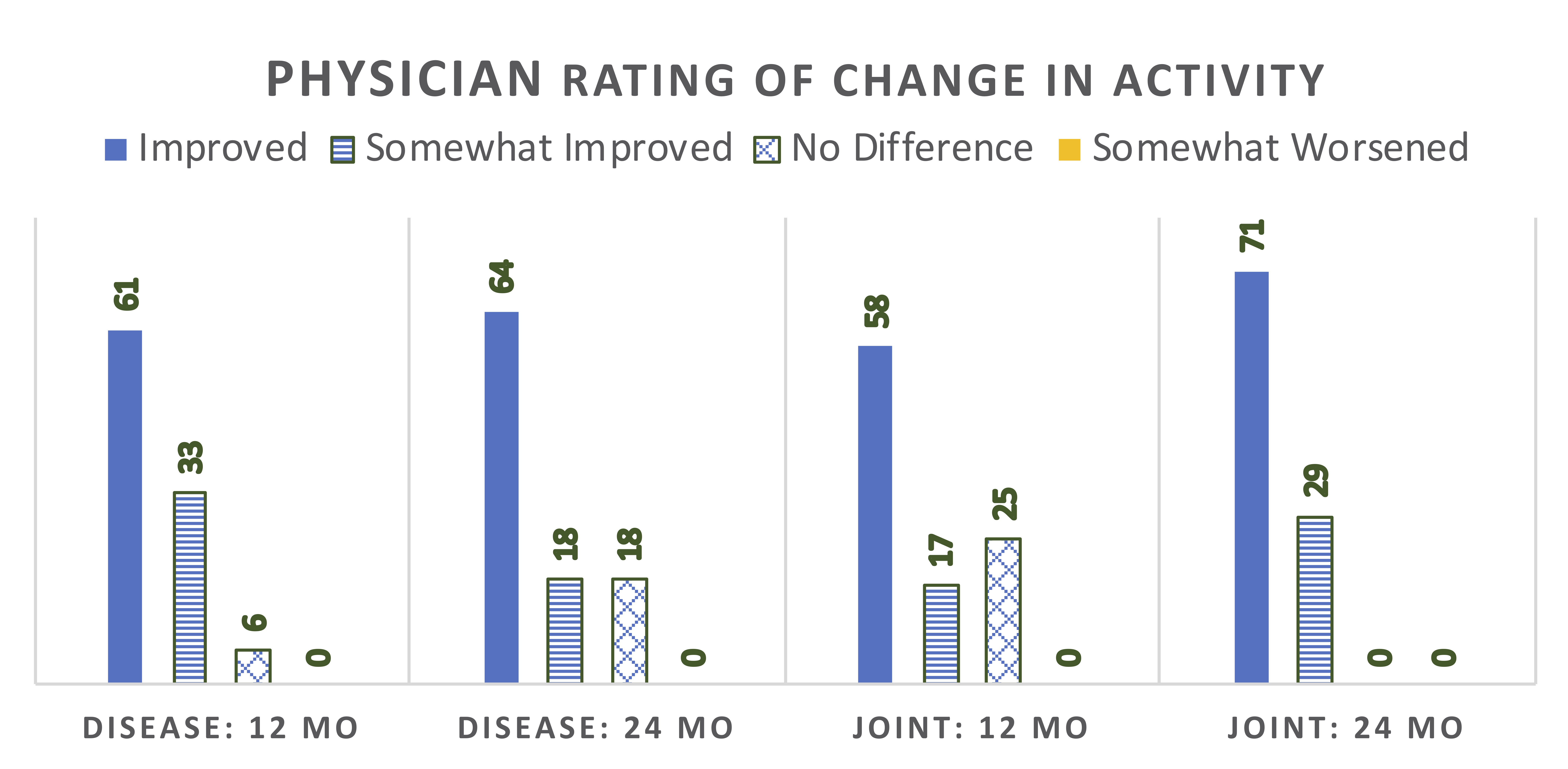
Median scores for physician global assessment of disease activity and skin activity measures all declined by 12 months, and then stabilized or further declined (Figure 1). At 12 months, 17 (94%) patients were rated as improved in activity status compared to baseline (Figure 2). At 24 months, 9 of 11 patients (82%) on ABA were rated as improved. Among the 12 patients with baseline joint involvement, 9 were rated as having improved joint status at 12 months (Figure 2). During ABA treatment, 10 patients were able to discontinue GC (71% of patients on GC).
Eight patients had an adverse event (AE); 7 were on concomittant medications. AE included fatigue, moodiness, behavioral change, and one infection (upper respiratory infection). No laboratory abnormalities were attributed to ABA. Two patients discontinued ABA because of mood and/or behavioral issues.
Conclusion: ABA was found to be a safe and effective treatment for jLS patients who previously failed MTX and/or MMF. There were no serious AE. Patients treated with ABA had a decrease in both skin and joint activity. Seventy percent of GC treated patients were able to discontinue GC. Prospective studies should be done to further evaluate ABA’s efficacy, potentially as stand-alone therapy and/or in combination with DMARDs.
All of the patients received other jLS treatment prior to being treated with abatacept: 17 were treated with methotrexate, 16 with mycophenolate mofetil, and 17 with glucocorticoids. The median duration of these treatments are listed. The median duration of abatacept treatment for the patients during their study follow_up is shown. ABA:abatacept; ANA: anti_nuclear antibody; CK: creatine kinase; ECM: extracutaneous manifestations; F: female; GC: glucocorticoid; IQR: interquartile range; MMF: mycophenolate mofetil; MTX: methotrexate
Skin Activity measures were mLoSSI and LSCAM. Physician global assessment were of activity (PGA_A) and damage (PGA_D). mLoSSI sums 3*disease extension + erythema + skin thickening across affected sites, LSCAM sums same variables without weighting disease extension, and also sums violaceous color, waxy white/ yellow, and tactile warmth.
LSCAM: Localized scleroderma cutaneous activity measure
mLoSSI: modified localized scleroderma severity index
PGA_A = Physician global assessment of disease activity
PGA_D = Physician global assessment of disease damage
To cite this abstract in AMA style:
Li S, Ishaq S, Buckley M, Torok K, Edelheit B, Ede K, Rabinovich C. Abatacept Treatment Reduces Cutaneous and Joint Activity in Juvenile Localized Scleroderma [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 4). https://acrabstracts.org/abstract/abatacept-treatment-reduces-cutaneous-and-joint-activity-in-juvenile-localized-scleroderma/. Accessed .« Back to 2020 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/abatacept-treatment-reduces-cutaneous-and-joint-activity-in-juvenile-localized-scleroderma/