Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Despite effective treatments, many patients (pts) with rheumatoid arthritis (RA) have inadequate responses to biologic DMARDs (bDMARD-IR), highlighting an unmet need. It is unclear whether prior bDMARD use affects efficacy of the oral, selective JAK-1 inhibitor filgotinib (FIL). This analysis explored the clinical response to FIL in bDMARD-IR pts stratified by mode of action (MOA) and number of prior bDMARDs.
Methods: The global, phase 3 FINCH-2 (NCT02873936) study treated 448 bDMARD-IR pts with active RA.1 Pts were randomized 1:1:1 to once-daily FIL 200 mg, FIL 100 mg, or placebo (PBO) for 24 weeks. Efficacy was assessed by percent of pts achieving low disease activity (LDA) or remission at week (W)24 as measured by CDAI and DAS28(CRP) stratified by number and MOA of prior bDMARDs. Comparisons were not adjusted for multiplicity. Non-responder imputation was used.
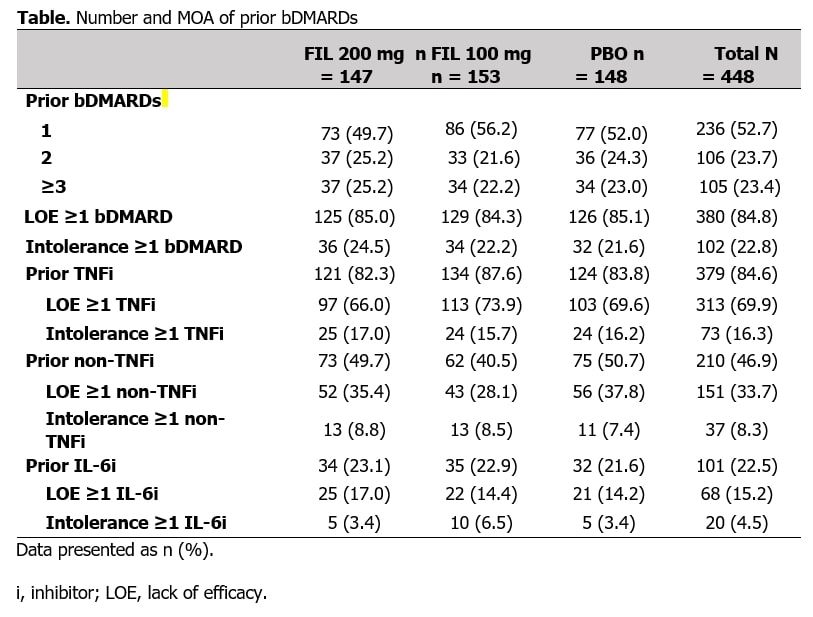
Results: In total, 448 bDMARD-IR pts were included, 105 with prior experience with ≥3 bDMARDs (Table). At W24, pts receiving FIL were in LDA at a higher proportion vs PBO, irrespective of number of prior bDMARDs or MOA (Figure 1). For pts receiving FIL 200 vs PBO, DAS28(CRP) ≤3.2 was achieved at W24 by 52% vs 26%, 51% vs 22%, and 38% vs 9% of pts with 1, 2, or ≥3 prior bDMARDs, respectively, and 49% vs 21% and 50% vs 13% of pts exposed to TNF or IL-6 inhibitors; for all subgroups, rates were significantly higher vs PBO (Figure 1). Delta between FIL 200 mg and PBO was maintained irrespective of number or type of prior bDMARDs. At W24, pts receiving FIL achieved remission at numerically higher rates vs PBO (Figure 2). For pts receiving FIL 200 mg vs PBO, DAS28(CRP) < 2.6 was achieved at W24 by 36% vs 14%, 30% vs 14%, and 22% vs 6% of pts with 1, 2, and ≥3 prior bDMARDs, respectively, and 31% vs 14% and 29% vs 9% of pts exposed to TNF or IL-6 inhibitors (Figure 2). Delta between FIL 200 mg and PBO was maintained irrespective of number or type of prior bDMARDs. Treatment-emergent adverse events across subgroups were consistent with overall study population.
Conclusion: Treatment with FIL vs PBO led to higher rates of LDA and remission in pts with IR to IL-6 or TNF inhibition, or to 1, 2, or ≥3 prior bDMARDs, with a similar safety profile to the overall study population. A significantly higher proportion of pts overall receiving FIL 200 mg vs PBO were in LDA at W24. Improved efficacy of FIL vs PBO in pts who previously failed multiple bDMARDs indicates distinct benefits of selective JAK-1 inhibition with FIL.
- Genovese, et al. JAMA 2019;322(4):315–25.
To cite this abstract in AMA style:
Gottenberg J, Buch M, Caporali R, Wright G, Takeuchi T, Kalunian K, Pechonkina A, Guo Y, Rao S, Tan Y, Besuyen R, Genovese M. A Subgroup Analysis of Low Disease Activity and Remission from Phase 3 Study of Filgotinib in Patients with Inadequate Response to Biologic DMARDs [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/a-subgroup-analysis-of-low-disease-activity-and-remission-from-phase-3-study-of-filgotinib-in-patients-with-inadequate-response-to-biologic-dmards/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-subgroup-analysis-of-low-disease-activity-and-remission-from-phase-3-study-of-filgotinib-in-patients-with-inadequate-response-to-biologic-dmards/