Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Rituximab in combination with methotrexate (MTX) is indicated for the treatment of adult patients with moderate to severe active rheumatoid arthritis (RA) who have an inadequate response to tumor necrosis factor therapies (TNF-IR). A structured systematic assessment of a drug’s benefit-risk profile is useful to inform decisions at each developmental milestone, and could meaningfully inform multiple stakeholders, physicians, patients, payors and researchers on the trade-offs between benefits and risks during the lifecycle of a drug product. The objective of this post-hoc analysis was to apply a structured descriptive approach to compare the benefit-risk profile of rituximab from the REFLEX 24-week placebo (PBO)-controlled trial and to produce a graphical representation of this profile in TNF-IR patients with RA.1
Methods: Presentations of data were based on the Benefit Risk Assessment Tool Framework developed for pharmaceutical benefit-risk decision-making in drug development and post-approval settings.2,3 Key benefits and risks of rituximab were identified and organized in a hierarchical manner to construct a ‘value tree’ as a basis for potential treatment benefits and risks. Absolute differences in efficacy outcome measures and safety event rates between (rituximab+MTX) and (PBO+MTX) treated patients were calculated to summarize key benefit-risk metrics and then presented graphically in a descriptive manner.
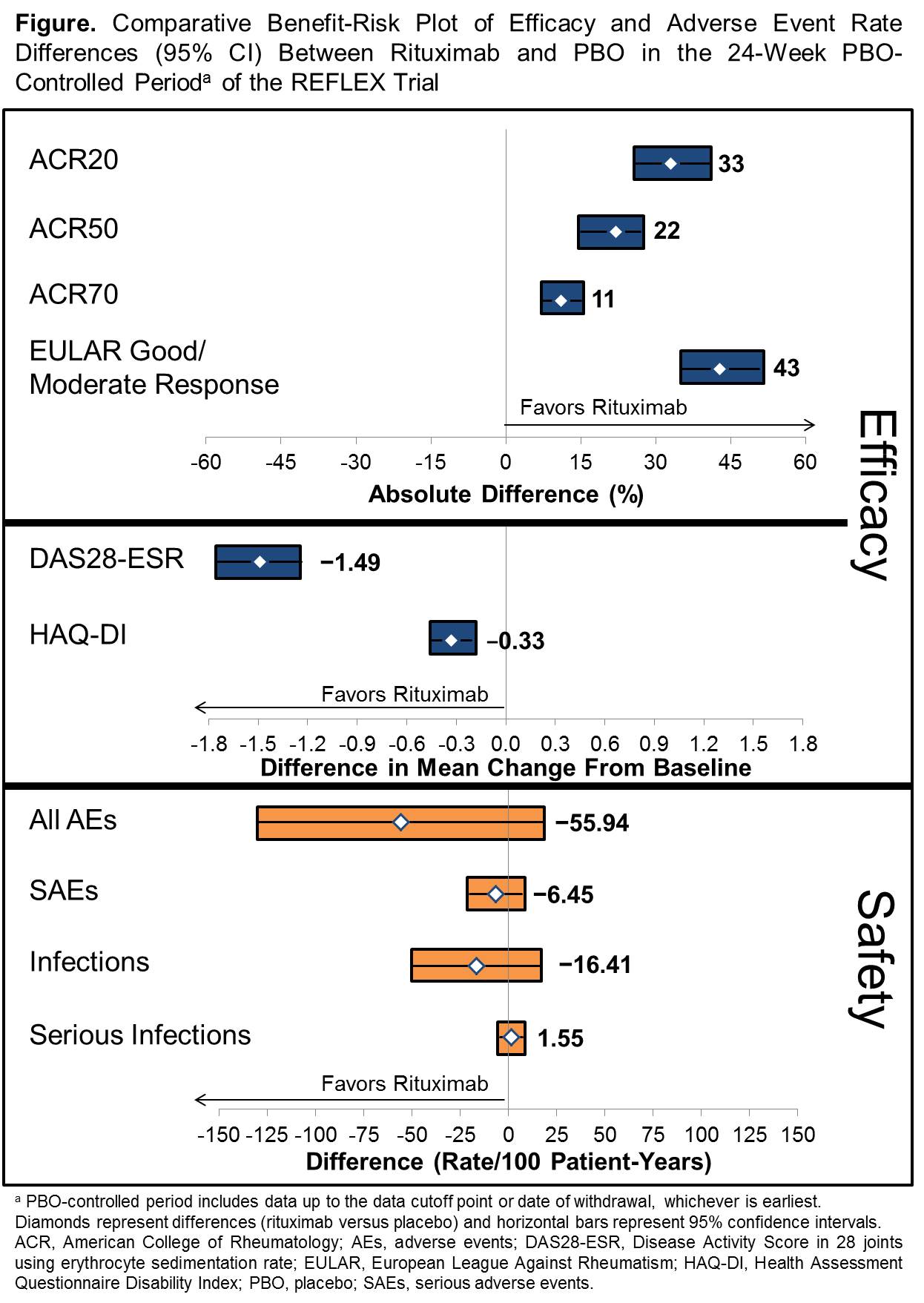
Results: Key benefits of rituximab treatment in TNF-IR patients identified included measures of clinical response (ACR and EULAR response and DAS28-ESR) and patient-oriented outcomes (Health Assessment Questionnaire Disability Index [HAQ-DI]). Safety assessments included rates of overall adverse events (AEs), serious AEs, all infections and serious infections. Mean (95% CI) efficacy differences and adverse event rate differences between the rituximab and PBO arms of the 24-week trial were calculated and displayed in the Figure. Overall, patients receiving rituximab treatment demonstrated meaningful clinical and statistically significant improvement compared with PBO in ACR20/50/70 response rates, EULAR response rates, DAS28-ESR and HAQ-DI, with no meaningful differences in safety events including serious AEs and infections.1
Conclusion: A graphical representation of key benefits and risks was generated to present the positive benefit-risk profile of rituximab in TNF-IR patients with RA. Future applications of this structured approach could include interpretation of the benefit-risk profile of longer term outcomes and of subpopulations of patients with RA receiving rituximab or a similar structured assessment of other drugs in a consistent manner.
.References:
1. Cohen SB, et al. Arthritis Rheum. 2006;54:2793-806.
2. Levitan BS, et al. Clin Pharmacol Ther. 2011;89:217-24.
3. Coplan PM, et al. Clin Pharmacol Ther. 2011;89:312-5.
Disclosure:
A. John,
Genentech, Inc,
3;
G. Quartey,
Genentech, Inc,
3;
P. B. Lehane,
Roche Products Ltd,
3;
N. Mairon,
F. Hoffmann-La Roche Ltd,
3;
M. Schulte,
F. Hoffmann-La Roche Ltd,
3;
A. Shewade,
Genentech, Inc,
3;
C. Chung,
Genentech, Inc,
3;
D. Borie,
Genentech, Inc,
3.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-structured-approach-for-comparative-benefit-risk-assessment-of-rituximab-for-the-treatment-of-rheumatoid-arthritis/