Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Systemic lupus erythematosus (SLE) is an autoimmune disease resulting from an interaction of genetic, epigenetic, hormonal, environmental and immunoregulatory influences. It has been well established that the HLA-DRB1*03:01 allele is a major risk factor in SLE; however, the mechanistic basis of the association is unclear. Our group has long proposed that in addition to presenting antigens, rheumatoid arthritis (RA)-associated HLA-coded molecules interact with a non-MHC receptor and trigger innate signaling events and lead to the manifestation of the disease. Here we report that, reminiscent of RA-associated HLA-DRB1 alleles, a lupus associated HLA-DRB1*03:01 epitope triggers cellular aberrations akin to known cellular events in SLE.
Methods: PMA-differentiated THP-1 cells and RAW 264.7 cells were treated with synthetic peptides corresponding to the third allelic hypervariable region (residues 65-79) of the DRb chain coded by three DRB1 alleles: *03:01, *04:01 and *04:02 (100 µg/mL) with or without recombinant mouse IFN-g (5 ng/mL). Cytotoxicity was determined by Lactate dehydrogenase (LDH) Kit (Roche). Intracellular ATP levels were measured with the ATPlite Luminescence Assay (PerkinElmer). Mitochondrial membrane potential and ROS were determined using the tetramethylrhodamine (TMRM) dye (Invitrogen), and MitoSOX Red (Molecular Probes), respectively, following the supplier’s protocol. Protein expression was determined by Western blotting. Griess Reagent assay (Promega) was used to estimate nitrite concentration. Cells were incubated for 2 hours with the following concentrations of ER-stress inhibitors: 5 mM 4-Phenylbutyric acid, (4-PBA); 10 μM 4μ8C; 25 μM AMG PERK 44; 10 μM Ceapin-A7.
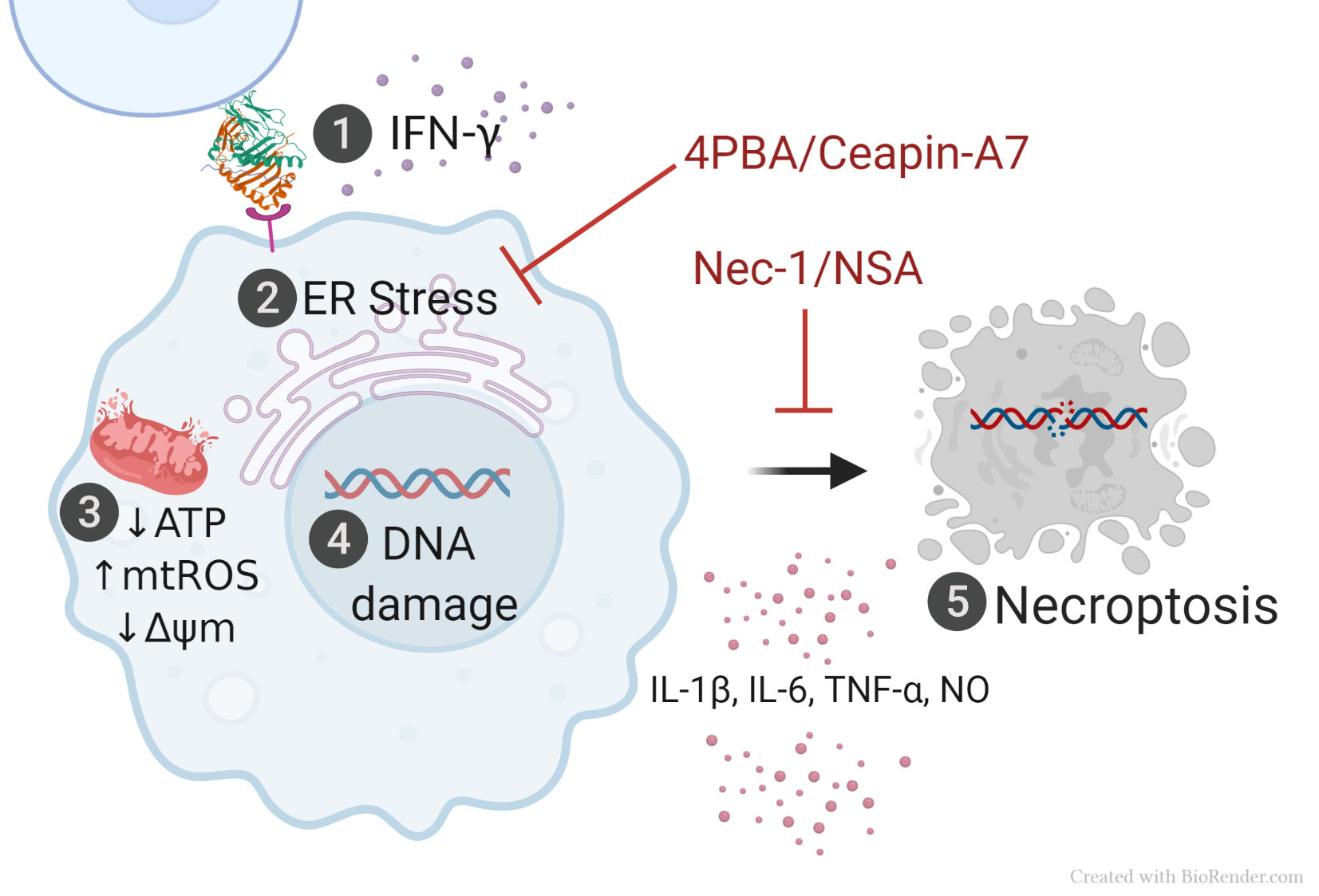
Results: In the presence of IFN-g, human THP-1 and mouse RAW 264.7 macrophage cell lines stimulated with 15-amino acid peptide sequence coded by HLA-DRB1*03:01 induced allele-specific signature SLE transcriptome, with activation of genes related to ER-stress and proteasomal degradation and triggered a cascade of cellular aberrations associated to SLE, including endoplasmic reticulum stress, mitochondrial dysfunction, DNA damage, cell death by necroptosis and release of proinflammatory cytokines. Cell death (p < 0.0001) and mitochondrial function assessed by intracellular ATP levels (p=0.0029), mitochondrial membraned potential (p < 0.0001) and mitochondrial ROS (p < 0.0001) were completely restored upon inhibition with chemical chaperone, 4-PBA. When investigating the possible ER-pathways involved, restoration of intracellular ATP levels pointed to the ATF6 pathway (p=0.008).
Conclusion: This study suggests a noncanonical, antigen presentation-independent mechanism of HLA-disease association in SLE and could lay new foundations for our understanding of key molecular mechanisms that trigger and propagate this devastating autoimmune disease. Translation of the findings to primary human macrophages derived from healthy controls and SLE patients could open the door to better understanding of the etiology and pathogenesis of SLE and in the long run, could potentially introduce new preventive, diagnostic and therapeutic solutions for the disease.
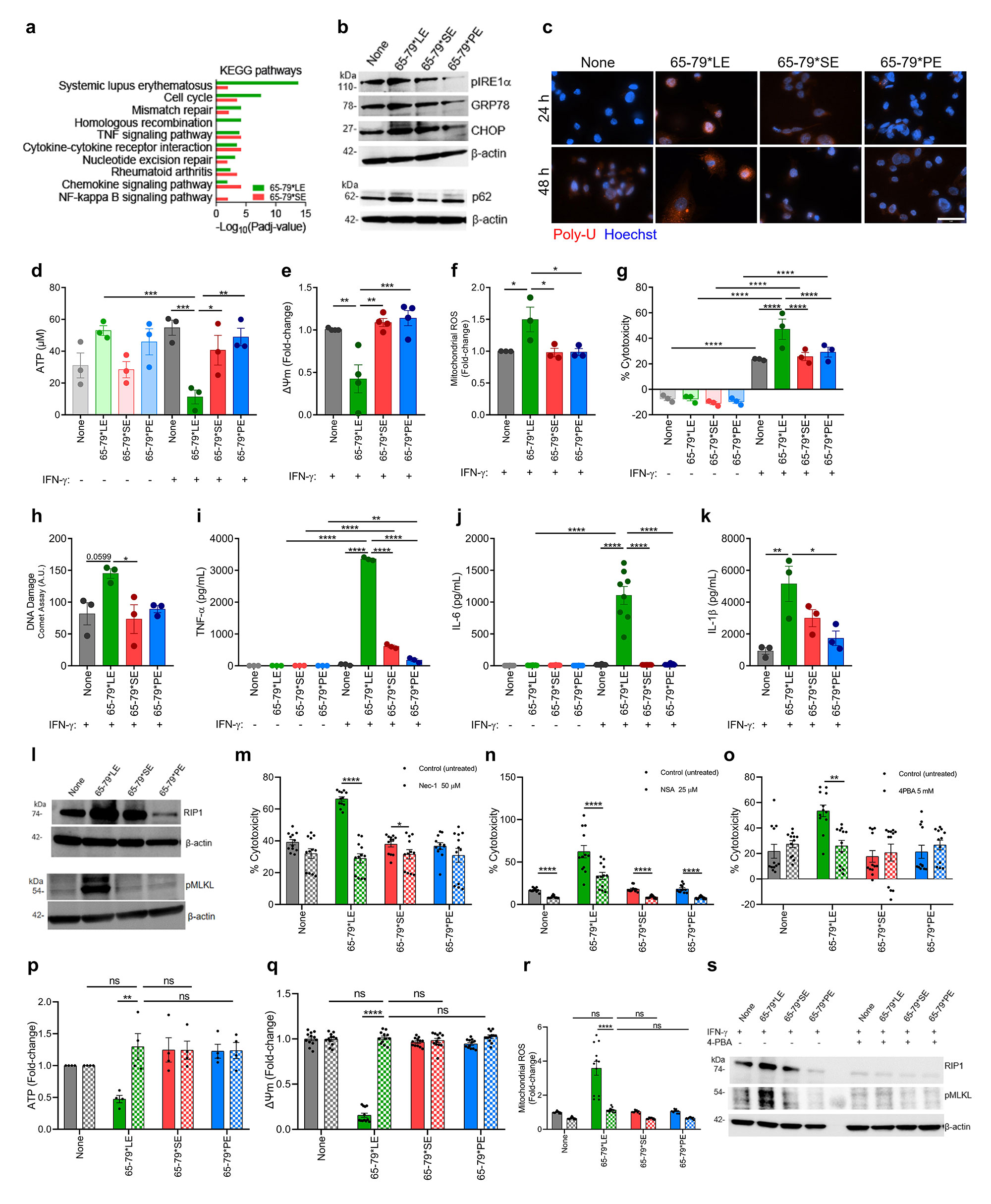
a, Notable KEGG pathway enrichment among 65-79*LE-upregulated DEGs, versus 65-79*SE.
Data are based on DEGs with a fold change >1.5 and Padj < 0.05. All data are based on 6 biological replicates in 2 independent experiments.
b, Representative immunoblots of ER stress markers (pIRE1-α, GRP78, CHOP) and proteasomal degradation marker (p62) in THP_1 macrophages in the presence of 65-79*LE, 65-79*SE, or 65-79*PE.
c, Representative immunocytochemistry images of poly-ubiquitinated protein accumulation (red) in RAW 264.7 macrophages. Scale bar = 2 μm.
d, Intracellular ATP levels reduction in allelic epitope-treated human THP_1 macrophages (n=3).
e, Mitochondrial membrane potential in allelic epitope-treated THP_1 macrophages, as determined by tetramethylrhodamine ethyl ester (TMRE), red (n=4).
f, Mitochondrial ROS in RAW 264.7 macrophages exposed to allelic epitopes in the presence of IFN-γ and measured by MitoSOX.
g, Cell death and h, DNA damage, quantified by the comet assay, in THP_1 cells exposed to different allelic epitopes (n=3).
i-k, Supernatant levels of the pro-inflammatory cytokines i, TNF-alpha j, IL-6 and k, IL_1β in THP_1 macrophages exposed to different allelic epitopes (n=3).
l, Immunoblots of Receptor-Interacting Protein 1 (RIP) and pMLKL in RAW 264.7 macrophages.
m, Cell death of RAW 264.7 macrophages in the presence (dotted bars) or absence (solid bars) of the RIP1 inhibitor Necrostatin_1 (50 μM) (n=3).
n, Cell death of RAW 264.7 macrophages in the presence (dotted bars) or absence (solid bars) of the MLKL inhibitor Necrosulfonamide (10 μM) (n=3).
o, Cell death in allelic epitope-treated RAW 264.7 macrophages in the presence (dotted bars) or absence (solid bars) of 4-PBA (n=3).
p, Intracellular ATP levels q, mitochondrial membrane potential and r, mitochondrial ROS levels in RAW 264.7 macrophages in the presence (dotted bars) or absence (solid bars) of 4-PBA (n=3).
s, Immunoblots of RIP1 and pMLKL in allelic epitope-treated RAW 264.7 macrophages in the presence (dotted bars) or absence (solid bars) of 4-PBA.
Blots are representative of 3 independent experiments. Data represent mean ± SEM. One-way (e,f,h,k) and two-way (d,g,I,j,m-r) ANOVA, * p < 0.05, ** p < 0.01, *** p < 0.001, ****p < 0.0001.
and necroptosis (5).
To cite this abstract in AMA style:
Miglioranza Scavuzzi B, Kaur B, Van Drongelen V, Mesquita-Ferrari R, Sawalha A, Miller F, Holoshitz J. A Short Allelic Epitope Coded by HLA-DRB1*03:01 Activates a Systemic Lupus Erythematosus Cell Aberration Cascade [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/a-short-allelic-epitope-coded-by-hla-drb10301-activates-a-systemic-lupus-erythematosus-cell-aberration-cascade/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-short-allelic-epitope-coded-by-hla-drb10301-activates-a-systemic-lupus-erythematosus-cell-aberration-cascade/