Session Information
Date: Monday, November 18, 2024
Title: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: ILD is associated with poor survival in patients with idiopathic inflammatory myopathies (IIM). Cyclophosphamide is often used in the management of myositis associated ILD (IIM-ILD). The EUROLUPUS (0.5g IV fortnightly for 6 doses) regime has previously been shown to be as effective as high dose regimes in lupus nephritis. This study aims to evaluate whether the low dose EUROLUPUS (LD) cyclophosphamide regime vs a high dose (HD) regime (600 mg/m2 IV every 4 weeks from day 0 to week 20) was associated with the same outcomes and better safety profiles in patients with IIM-ILD.
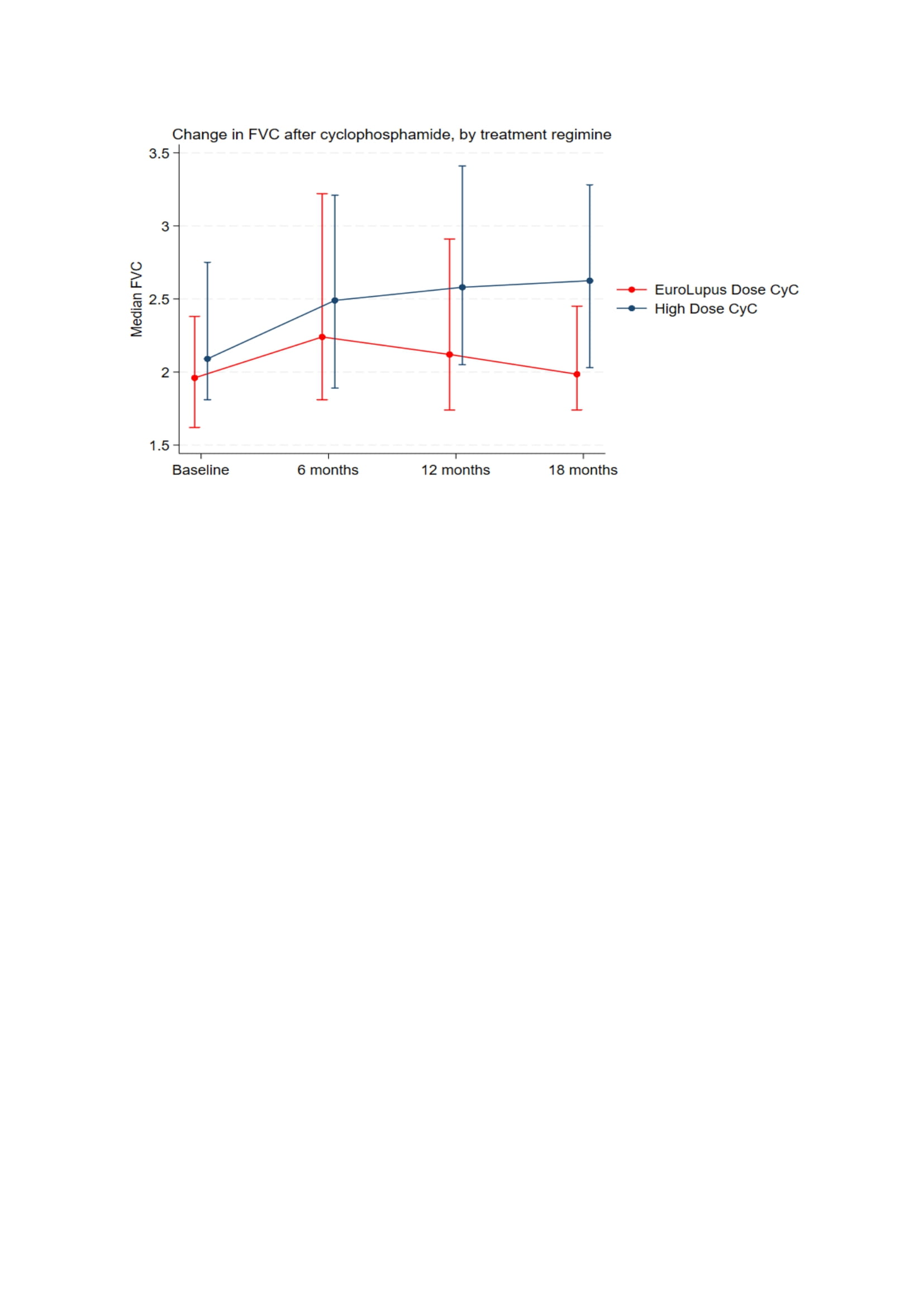
Methods: A retrospective analysis of notes was undertaken for patients attending the King’s Health Partners ILD service across two hospital sites (King’s College Hospital and Guy’s Hospital) with a diagnosis of ILD and myositis or ILD and myositis specific antibodies. Adult patients who received cyclophosphamide with at least 18 months of follow up lung function and HRCT data were analysed. Patients whose therapy was started on ITU were excluded. Descriptive statistics were used to compare the differences between the two regimes at baseline and follow up. Change in FVC and TLCO over 18 months was graphically compared at predefined time points.
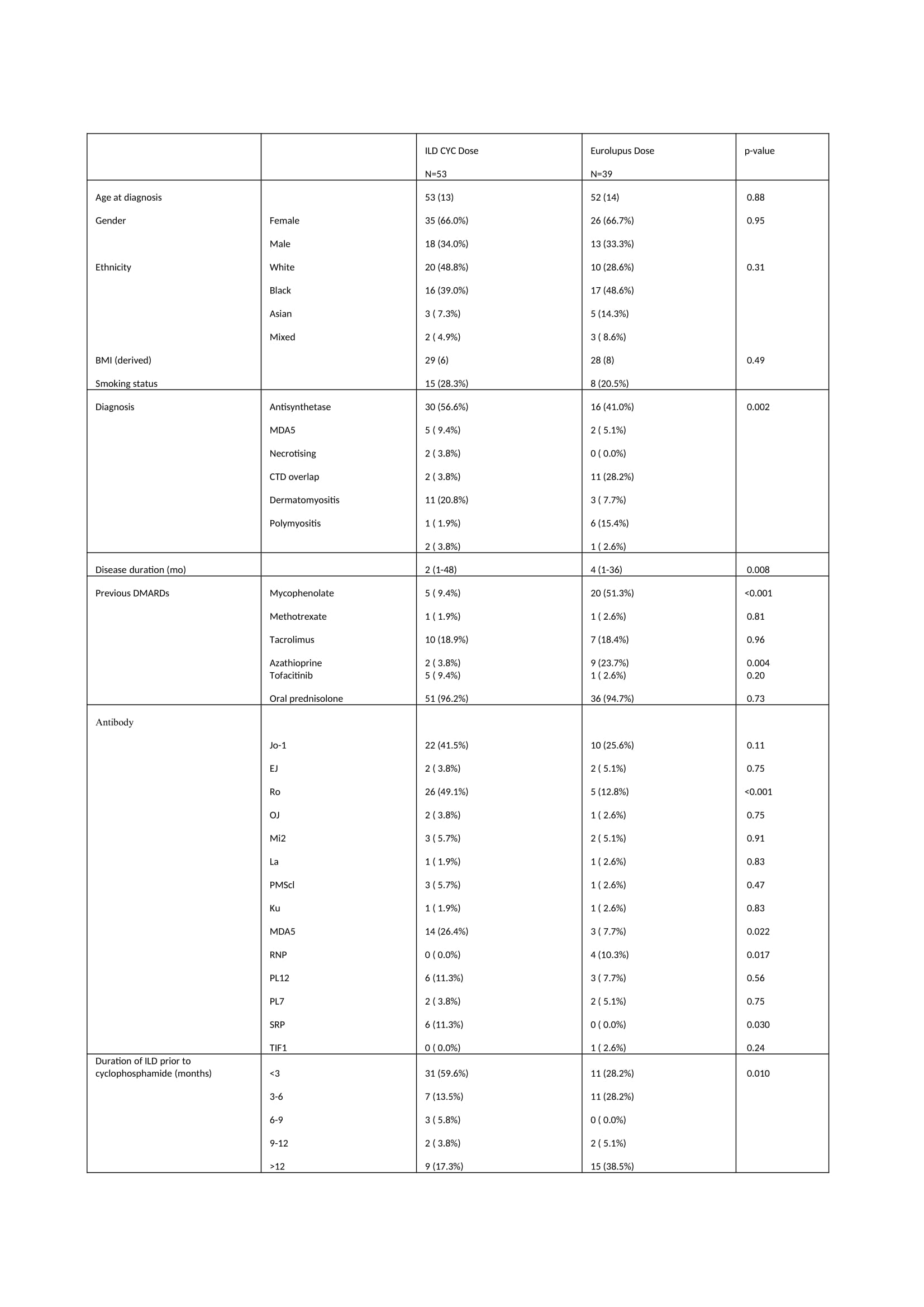
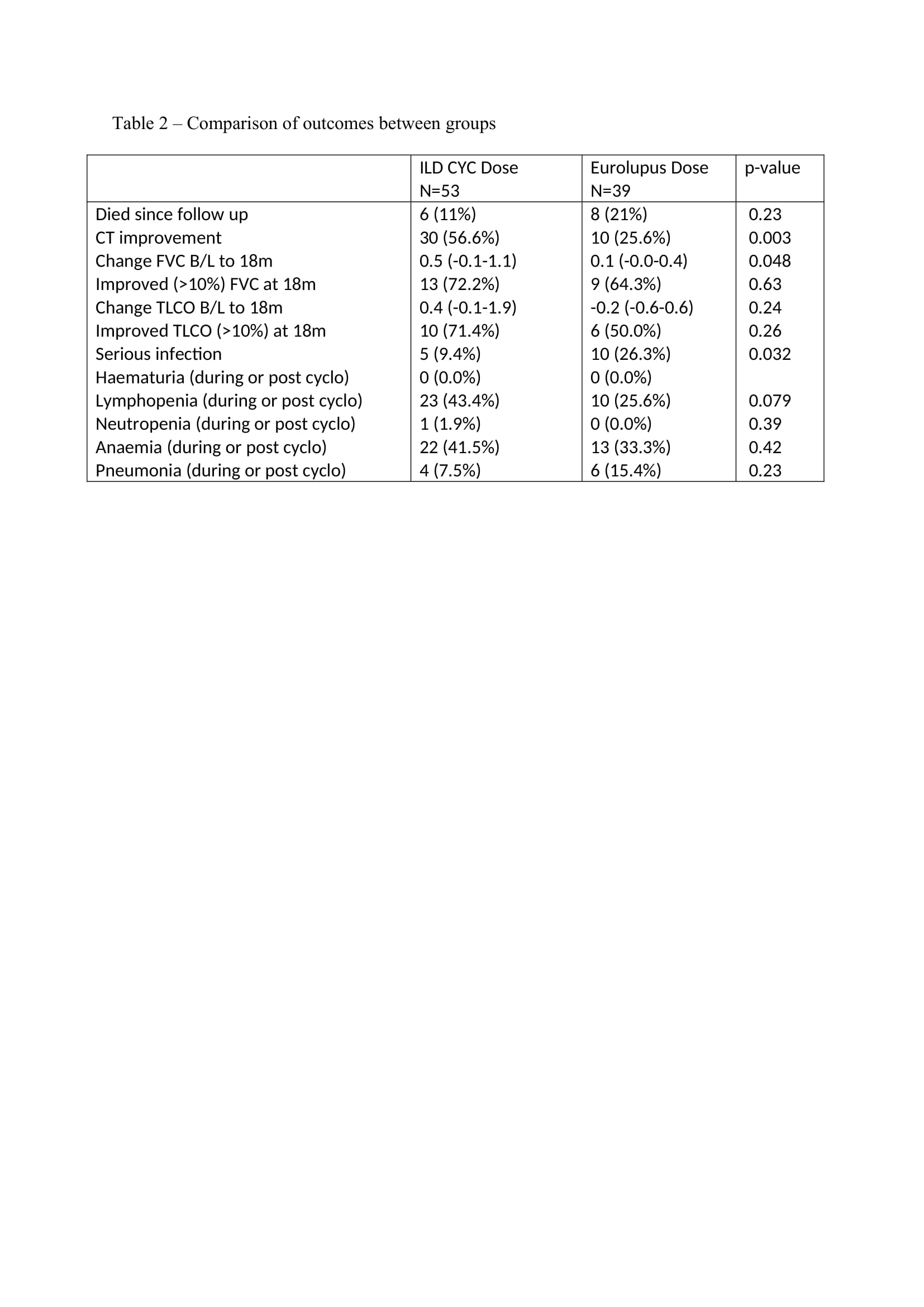
Results: 92 patients received cyclophosphamide for IIM-ILD. 53 (57.6%) patients received the HD regime vs 39 (42.3%) in the LD arm. The mean age was 53 (13). 61 (66.3%) patients were female. Anti-Jo-1 antibody was positive in 41.5% in the high dose group vs 25.6% in the low dose group (Table 1). All-cause mortality was 11% in the HD arm vs 21% in the LD arm (p=0.23). Change in FVC (L) from baseline to 18 months is presented in figure 1. At 18 months, the change from baseline was greater in the HD arm [median 0.5 (IQR:-0.1-1.1) vs 0.1 (-0.0-0.4) (p=0.048)]. Change in TLCO at 18 months was numerically higher in the HD arm, but did not reach statistically significant [0.4 (-0.1-1.9) vs -0.2 (-0.6-0.6) (p=0.24)]. 9.4% in the HD arm had a serious infection vs 26.3% in the LD arm (p=0.032). Lymphopenia was higher in the HD arm vs LD arm (43.4% vs 25.6%, p=0.079) (Table 2). Overall, Patients in the LD arm had a longer duration of ILD, more NSIP, less OP, more frequent Mycophenolate Mofetil use, more pulmonary hypertension, less frequent concomitant Rituximab and a significantly higher CK at baseline, (suggesting more severe/systemic disease).
Conclusion: HD cyclophosphamide was associated with a significant improvement in FVC vs LD cyclophosphamide at 18 months. All-cause mortality numerically favoured high dose cyclophosphamide and there were significantly fewer serious infections in the HD arm. Imbalances between the groups, in terms of severity of disease, may account for these observed differences. This study serves as an initial exploratory analysis for the use of low dose cyclophosphamide regimes in IIM-ILD but larger randomised trials would be needed to assess the efficacy and safety of this regime.
To cite this abstract in AMA style:
Ali S, Lawrence A, Bechman K, Patel A, biring S, Steer S, Mahto A, Myall C, Pollard L, Naqvi M, Holloway A, Salerno R, Dell'accio F, Agarwal S, Lams B, West A, Gordon P. A Retrospective Cohort Study Assessing Outcomes and Safety in Patients Receiving Low Dose vs High Dose Cyclophosphamide in Myositis Interstitial Lung Disease (ILD) [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-retrospective-cohort-study-assessing-outcomes-and-safety-in-patients-receiving-low-dose-vs-high-dose-cyclophosphamide-in-myositis-interstitial-lung-disease-ild/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-retrospective-cohort-study-assessing-outcomes-and-safety-in-patients-receiving-low-dose-vs-high-dose-cyclophosphamide-in-myositis-interstitial-lung-disease-ild/