Session Information
Date: Sunday, November 7, 2021
Title: RA – Diagnosis, Manifestations, & Outcomes Poster II: Miscellaneous Aspects of RA (0786–0812)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Patients (pts) with immune-mediated rheumatic diseases and an insufficient response to previous treatment with TNFα inhibitors (TNFαi) are frequently encountered in clinical practice. This study assessed the effectiveness of golimumab (GLM) and its impact on patient-reported outcomes in this population.
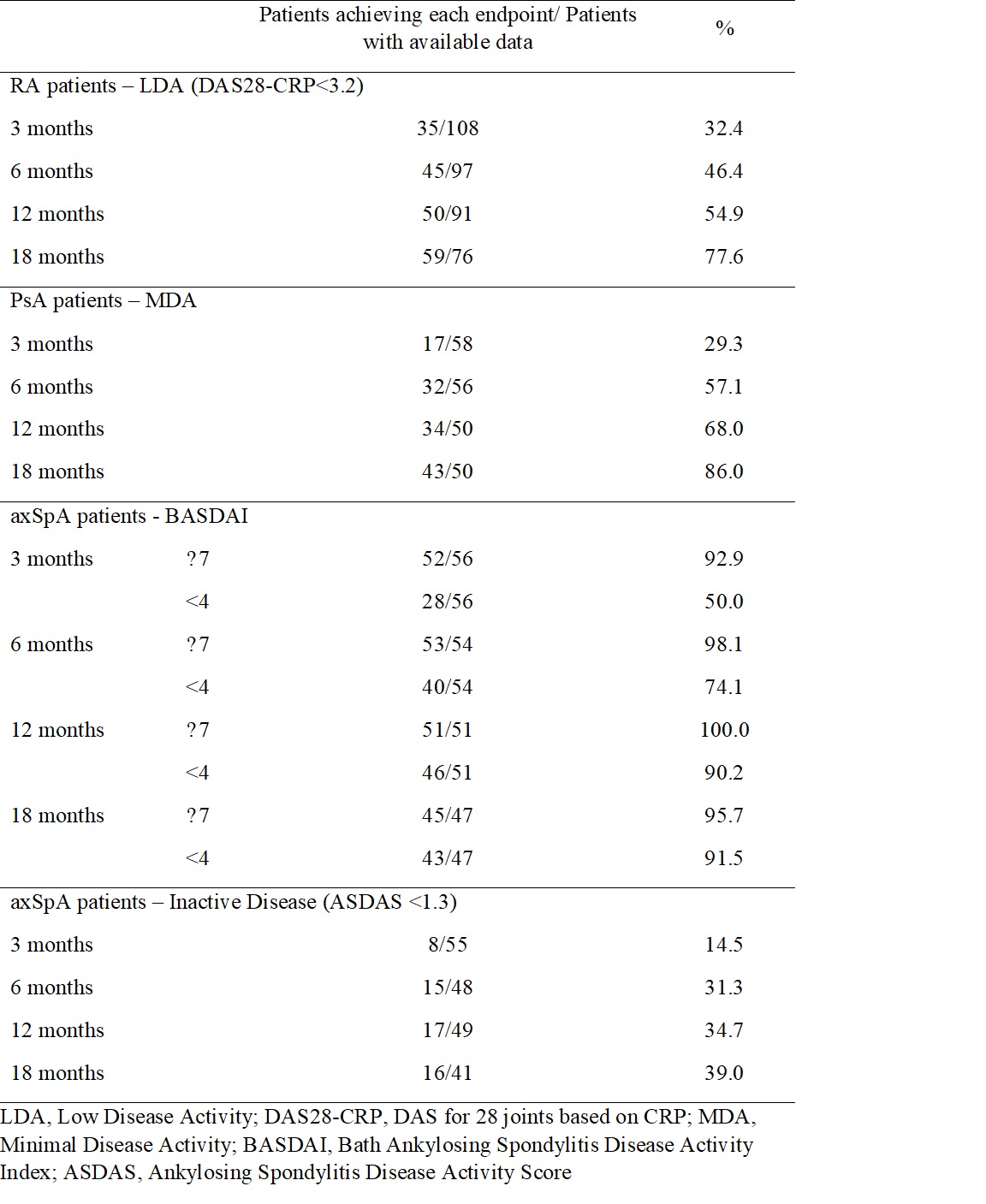
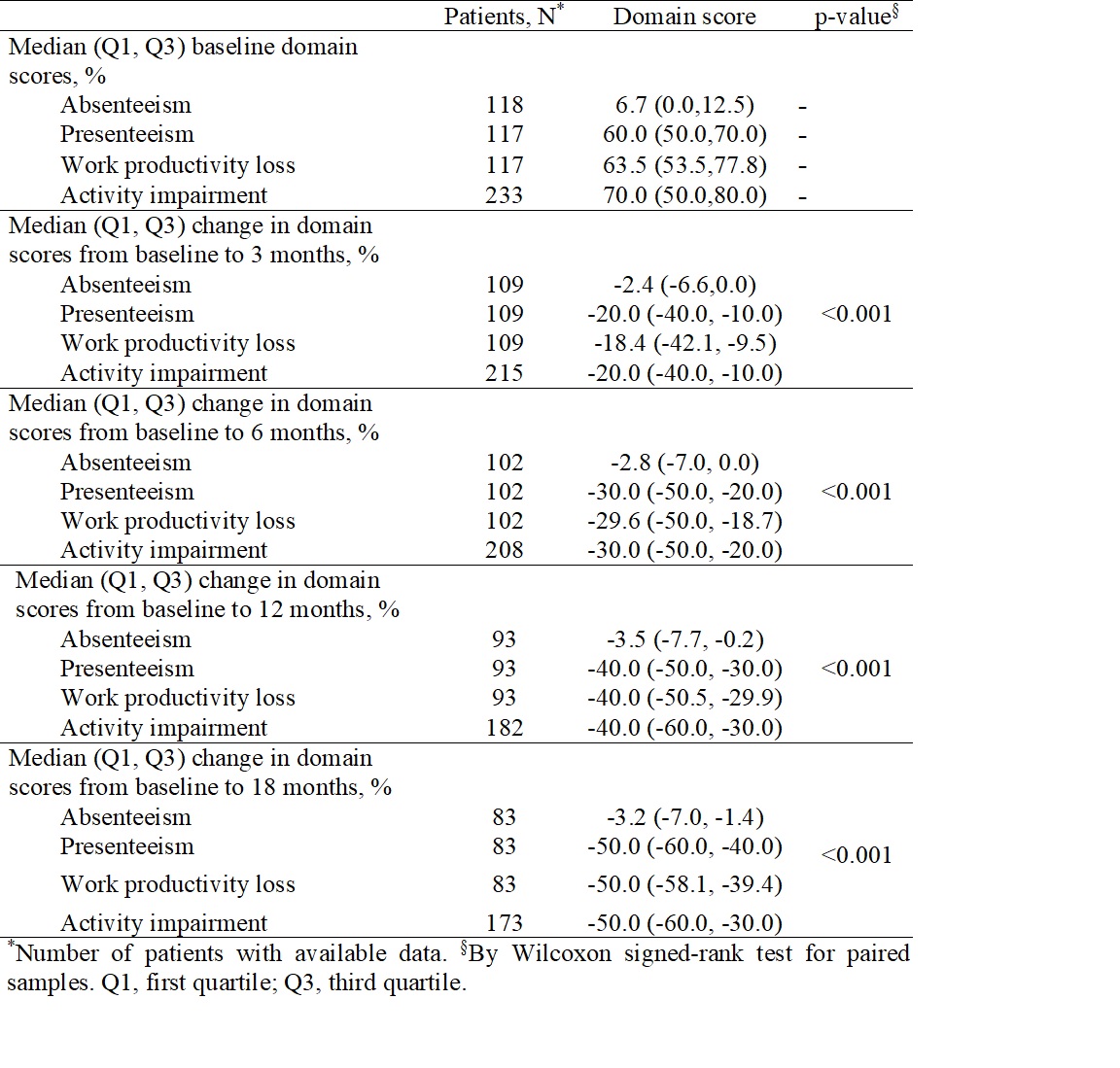
Methods: GO-BEYOND was a real-world, prospective, 18-month study conducted in 25 sites in Greece. Eligible pts had active RA, PsA, or axSpA and had failed previous treatment with one TNFαi due to secondary non-response (defined as disease relapse following ≥6 months of treatment), intolerability, or inconvenience. The primary objective assessed the proportion of pts with: RA attaining low disease activity (LDA; DAS for 28 joints based on CRP [DAS28-CRP] < 3.2); PsA attaining minimal disease activity (MDA; defined by MDA criteria); and, axSpA attaining moderate disease activity (Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] between 4 and 7) at 6 months. Disease activity, including inactive disease in axSpA pts (Ankylosing Spondylitis Disease Activity Score [ASDAS] < 1.3), was assessed at months 3, 12 and 18 as part of the secondary objectives, which also assessed work productivity and activity impairment (Work Productivity and Activity Impairment Questionnaire [WPAI]), QoL (EQ-5D-3L questionnaire), and healthcare resource utilization (HCRU) via a patient diary, at baseline (BL), 3, 6, 12, and 18 months in all indications. Drug persistence was evaluated using the Kaplan-Meier method.
Results: A total of 242 pts (RA: 117 [48.3%]; PsA: 63 [26.0%]; axSpA: 62 [25.6%]) were included, with a mean (SD) age of 55.1 (13.5). Most pts were female (173, 71.5%). The median (Q1-Q3) time from diagnosis to study entry was 3.6 (2.1-7.1) years. At BL, pts with RA had a median (Q1-Q3) DAS28-CRP of 4.8 (4.5-5.3), pts with PsA had a median (Q1-Q3) DAS28-CRP of 4.7 (4.3-5.1), and pts with axSpA had a median (Q1-Q3) BASDAI of 6.2 (4.7-6.9). Following 6 months of GLM treatment, 46.4% (45/97) of pts with RA achieved LDA and 57.1% (32/56) of pts with PsA achieved MDA; of pts with axSpA, 98.1% (53/54) achieved BASDAI≤7, 74.1% (40/54) achieved BASDAI < 4 and 31.3% (15/48) achieved inactive disease by ASDAS. Improvements in disease activity were also observed across each time point (3, 12 and 18 months) (Table 1). In the entire cohort (RA, PsA, and axSpA), the changes in all WPAI domain scores from BL to 3, 6, 12, and 18 months were significant (p < 0.001 all comparisons; Table 2), as were the respective changes in the EQ-5D-3L UK index score (Table 3). Of all pts, ~90.0% had disease-related laboratory tests performed and ~20.0% visited specialist physicians during the observation period; no hospitalizations were recorded. Finally, the persistence rates at 3, 6, 12 and 18 months were 93.7%, 89.0%, 85.1% and 85.1%.
Conclusion: In a real – world setting in Greece, pts with active RA, PsA or axSpA, treatment with GLM over 18 months resulted in clinically relevant improvements in disease activity, while a high persistence rate was observed. Significant improvements in work productivity, activity impairment, and QoL were observed from BL to 3, 6, 12 and 18 months. HCRU was mostly driven by laboratory tests.
Disclosures: This study was funded by MSD Greece
To cite this abstract in AMA style:
Athanassiou P, Psaltis D, Georgiadis A, Katsifis G, Theodoridou A, Gazi S, Sidiropoulos P, Tektonidou M, Bounas A, Kandyli A, Vounotrypidis P, Sakellariou G, Vassilopoulos D, Huang Z, Petrikkou E, Boumpas D. A Real-world Prospective Observational Study of the Effectiveness of Golimumab in Adult Greek Patients with RA, PsA and Axial SpA and Inadequate Response to Initial TNFα Inhibitor Therapy [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/a-real-world-prospective-observational-study-of-the-effectiveness-of-golimumab-in-adult-greek-patients-with-ra-psa-and-axial-spa-and-inadequate-response-to-initial-tnf%ce%b1-inhibitor-therapy/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-real-world-prospective-observational-study-of-the-effectiveness-of-golimumab-in-adult-greek-patients-with-ra-psa-and-axial-spa-and-inadequate-response-to-initial-tnf%ce%b1-inhibitor-therapy/