Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Long term use of conventional synthetic DMARDs (csDMARDs) and biologics in RA has clinical risks including infection and malignancy. Patients with chronic RA remain interested in the question of medication tapering per our previous survey-based study within the RHEUmatoid Arthritis Medication TAPering (RHEUMTAP) cohort. Few RCTs and retrospective studies have systematically studied clinical outcomes of medication tapering in RA. In this study, we prospectively compared the clinical outcomes of different medication tapering groups within the RHEUMTAP cohort.
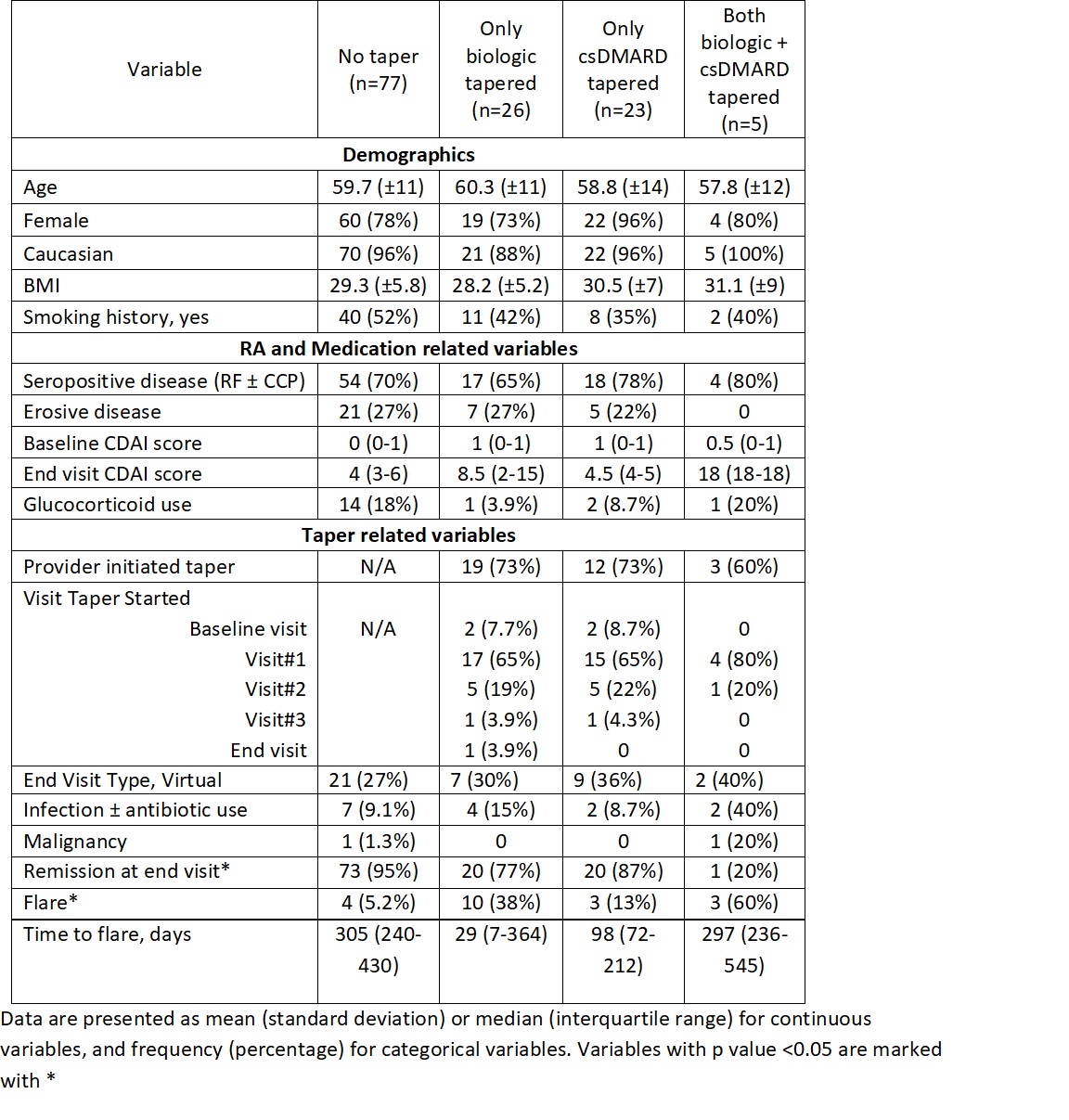
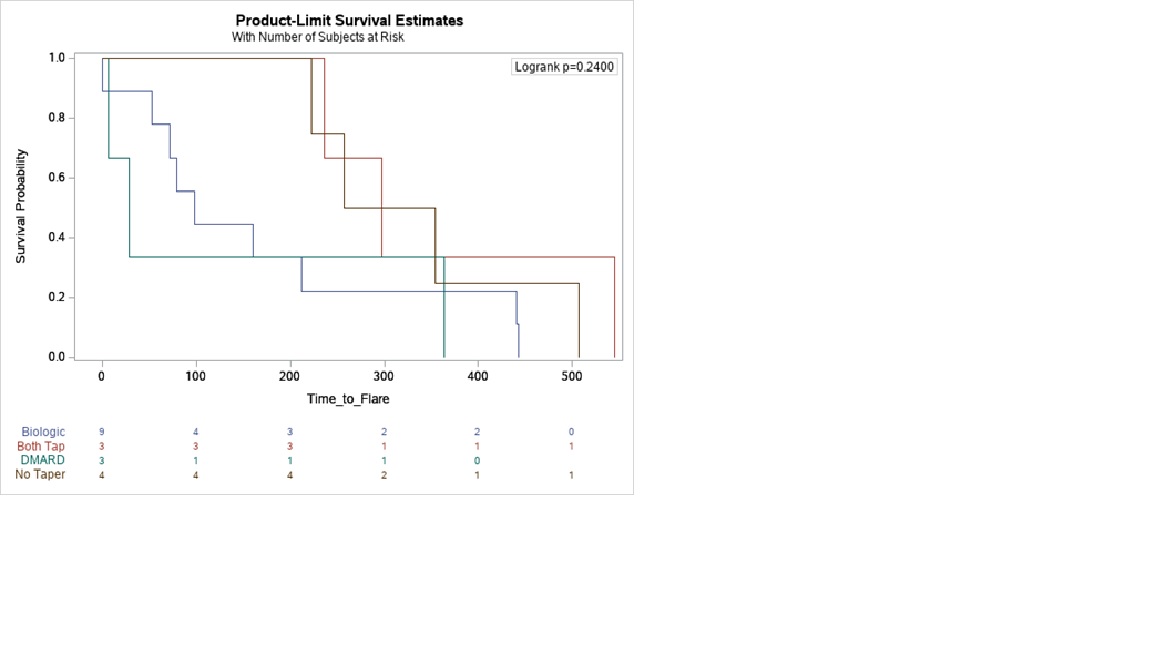
Methods: RHEUMTAP is a 2 year prospective cohort of patients with RA in sustained remission on biologics seen at Allegheny Health Network Rheumatology between 11/30/2018 and 11/30/2020 with at least 6 months follow up. Biologic tapering regimens were pre-determined based on provider consensus. Manual electronic health record (EHR) review was performed to confirm sustained remission on biologic therapy defined as CDAI<2.8, stable RA medication use, and absence of flares for a period of at least 6 months. Taper meant a reduction in biologic and/or csDMARD dose, frequency, or discontinuation. Primary outcome was proportion of flares at the end of study period, and secondary outcome was time to flare. Flare was defined as provider diagnosed worsening RA requiring initiation, change or increase in therapy. The 4 groups of comparison were: no taper, only biologic tapered, only csDMARD tapered, and both biologic and csDMARD tapered. Statistical analyses were completed using SAS Enterprise Guide 7.15 HF3 (SAS Institute, Inc, Cary, NC). Multivariable logistic regression analyses were used to estimate adjusted odds of flare. Kaplan Meier survival plots were created to determine the time to flare utilizing the log-rank test to determine significance.
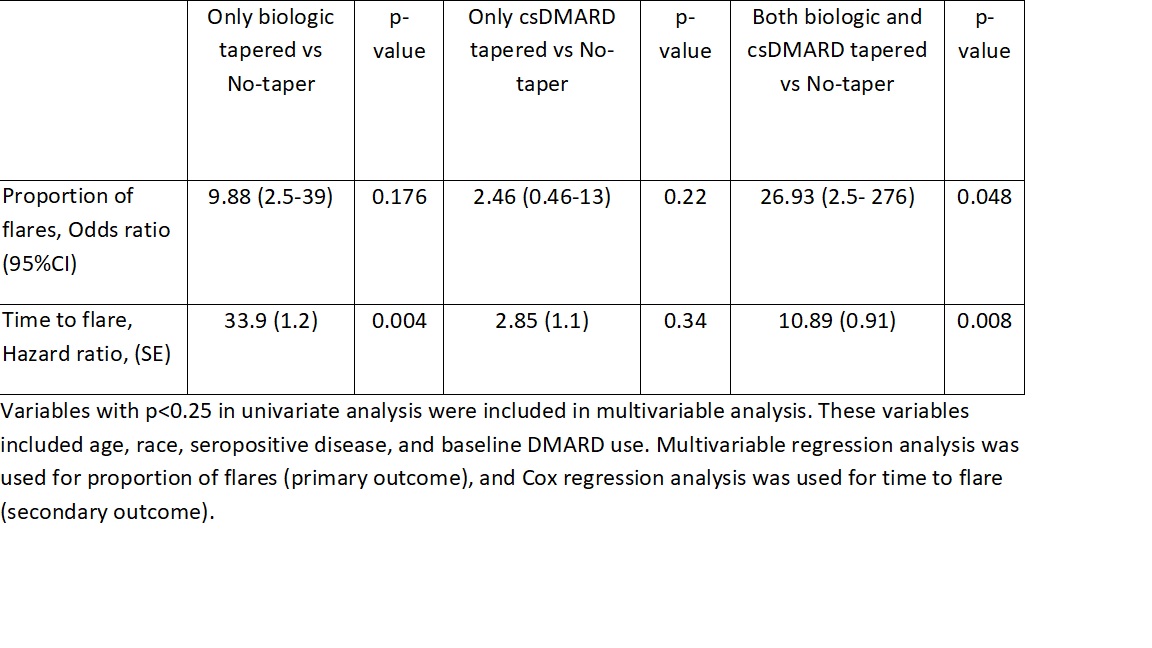
Results: The RHEUMTAP cohort included 346 patients of which 131 patients were confirmed to have sustained remission on biologics +/- csDMARDs based on manual EHR review. Of these 131 patients, 54 patients underwent a medication taper. Flare was experienced by 20 (15%) patients during follow up period, of which 16 were in the taper groups and 4 in the no-taper group. There were no significant differences in characteristics between the groups (table 1). The most frequently tapered biologics were etanercept and adalimumab, and most frequent csDMARD tapered was methotrexate. Patients undergoing any taper (all groups combined) overall were 7.6 times more likely to experience a flare in the follow-up period compared to those not tapered (OR 7.68, 95% CI 2.4-24, p=0.0006). The both biologic and csDMARD tapered group was more likely to experience a flare (OR 26.93, 95% CI 2.5- 276, p< 0.048) and have a shorter time to flare (HR 10.89, SE 0.91, p=0.008) compared to the no-taper group (table 2).
Conclusion: RHEUMTAP is the first real-world prospective cohort study to report the outcomes of different medication tapering groups in RA in sustained remission. In our cohort, patients who tapered both biologics and csDMARDs were more likely to experience a flare and have a shorter time to flare. Larger studies are required to confirm our findings given our wide confidence intervals.
To cite this abstract in AMA style:
Tageldin M, Attur M, Wilson N, Schorr R, Sharma T. A Real-World 2-Year Prospective Study of Medication Tapering in Patients with RA in Sustained Remission in the RHEUmatoid Arthritis Medication TAPering (RHEUMTAP) Cohort [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/a-real-world-2-year-prospective-study-of-medication-tapering-in-patients-with-ra-in-sustained-remission-in-the-rheumatoid-arthritis-medication-tapering-rheumtap-cohort/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-real-world-2-year-prospective-study-of-medication-tapering-in-patients-with-ra-in-sustained-remission-in-the-rheumatoid-arthritis-medication-tapering-rheumtap-cohort/