Session Information
Date: Monday, November 6, 2017
Title: Osteoarthritis – Clinical Aspects Poster I: Clinical Trials and Interventions
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: CR4056 is a novel imidazoline-2 receptor (I2R) ligand endowed with potent analgesic activities in several and diverse animal models of nociceptive and neuropathic pain, by innovative modulation of the monoaminergic descending inhibitory pathway (Li JX, Pharmacol Ther 2017). The present proof-of-concept study was undertaken to investigate the efficacy and safety of CR4056 in patients with knee osteoarthritis (OA) pain.
Methods: This was a multicenter, prospective, randomized, placebo-controlled, double-blind, parallel group design trial (EudraCT n. 2015-001136-37). Patients with knee OA (ACR clinical and radiological criteria, Kellgren & Lawrence grade 2/3) and moderate to severe pain (score ≥50 on the 0-100 normalized WOMAC pain subscale) were randomized in a 2:1 ratio to a pharmacologically effective dose of oral CR4056 (100 mg b.i.d. in women and 200 mg b.i.d. in men, to assure similar exposure due to slight gender differences in pharmacokinetics) or matching placebo for 14 days. Intention-to-treat (ITT: Worst-Case approach for non-completers) changes in WOMAC pain (primary endpoint) were analysed by the Wilcoxon test in the overall study population and in different OA phenotypes, including patients with a neuropathic pain component (as per the painDETECT questionnaire), or obesity (BMI≥27.5 kg/m2, i.e. the WHO threshold for pre-obesity).
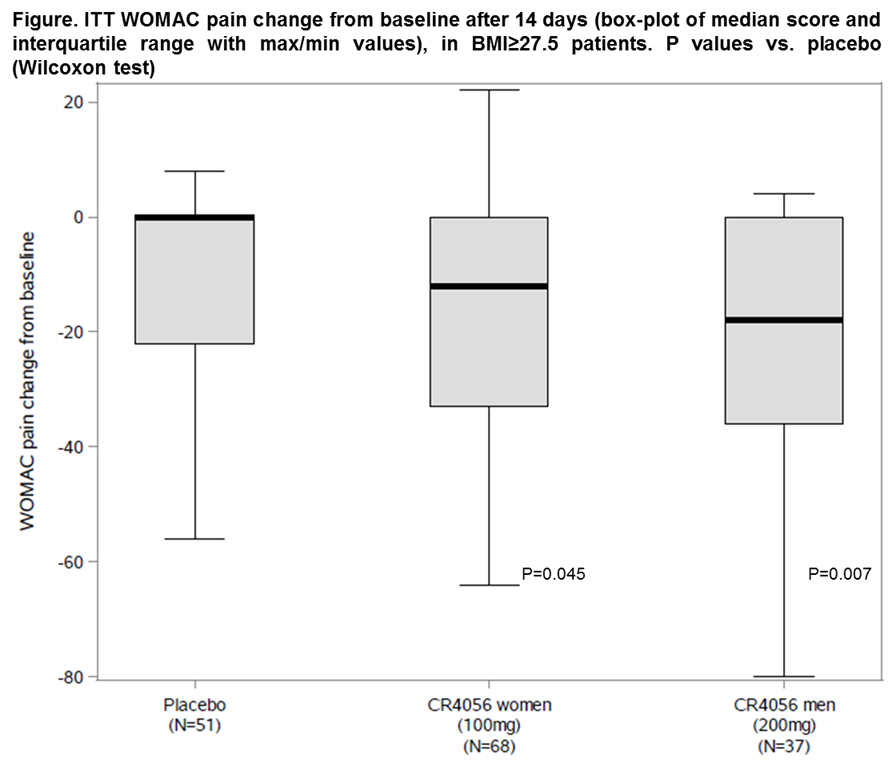
Results: A total of 213 patients were blindly randomized at 20 sites in Poland and UK: 92 women to CR4056 100 mg b.i.d., 52 men to 200 mg b.i.d. and 69 overall to placebo, with median (range) WOMAC pain baseline scores of 61 (50;90), 58 (50;90) and 58 (50;84), respectively, that were similar across groups and OA phenotypes. Three patients withdrew with CR4056 100 mg b.i.d., 5 with 200 mg b.i.d. and 6 with placebo. There were no serious adverse events and treatments were equally well tolerated. CR4056 decreased WOMAC pain vs. placebo after only 14 days and with a similar pattern in the overall population and the selected OA phenotypes. While only 21/213 (9.9%) patients had a neuropathic pain component, 73.2% (156 out of 213) had BMI≥27.5 (mean 33.4). In these patients, CR4056 significantly decreased WOMAC pain (Figure) in a clinically relevant fashion: pooled CR4056 ITT median (range) change was -14 (-80;22) vs. 0 (-56;8) with placebo (P=0.011; n=105 and 51, respectively). Secondary pain and function outcomes followed a pattern consistent with the primary endpoint.
Conclusion: CR4056 is the first I2R ligand to show analgesic activity in humans. The compound was safe and effective in reducing knee OA pain in this very short phase II trial, especially in overweight and obese patients. This observation prompts longer-term trials and the exploration of possible links between the I2 pathway and the overweight or metabolic OA phenotype altered pain perception. Conversely, a neuropathic pain component had low prevalence in unselected knee OA patients in this study.
To cite this abstract in AMA style:
Rovati LC, Brambilla N, Blicharski T, Probert NJ, Vitalini C, Giacovelli G, Girolami F, D'Amato M. A Randomized, Placebo-Controlled, Double-Blind, Phase II Clinical Trial of the First-in-Class Imidazoline-2 Receptor Ligand CR4056 in Pain from Knee Osteoarthritis and Disease Phenotypes [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/a-randomized-placebo-controlled-double-blind-phase-ii-clinical-trial-of-the-first-in-class-imidazoline-2-receptor-ligand-cr4056-in-pain-from-knee-osteoarthritis-and-disease-phenotype/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-placebo-controlled-double-blind-phase-ii-clinical-trial-of-the-first-in-class-imidazoline-2-receptor-ligand-cr4056-in-pain-from-knee-osteoarthritis-and-disease-phenotype/