Session Information
Date: Tuesday, November 7, 2017
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy III: Biosimilars Therapy
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Rituximab (RTX) is a mAB indicated for the treatment of RA in patients with inadequate response to anti-TNF therapy. The current study compares the biosimilar GP2013 with the reference medicine approved in Europe, RTX-EU and in the US, RTX-US.
Methods: Eligible patients were randomized to GP2013, RTX-EU or RTX-US. The primary endpoint was the area under the serum concentration–time curve from study drug infusion to infinity (AUC0-inf). The read-out for primary and all key secondary analyses were performed at week 24 (results submitted for publication). Patients were then followed up to week 52.
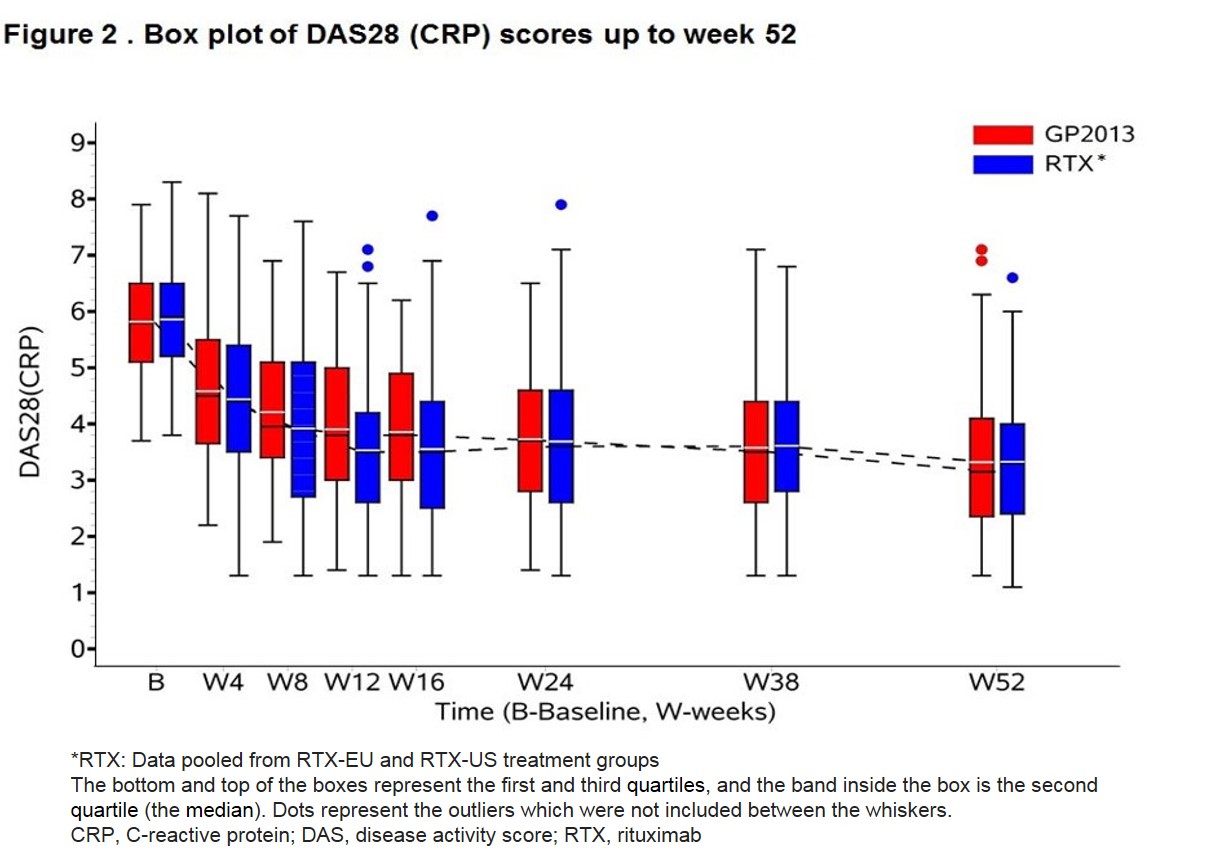
Results: A total of 312 patients (262 female and 50 male) were randomized. Demographics and baseline characteristics were comparable between the groups. The 90% CI of the ratio of the geometric mean were within the predefined range of 80-125% for the primary and key secondary pharmacokinetic and pharmacodynamic (PD) endpoints demonstrating three-way bioequivalence of GP2013, RTX-EU and RTX-US at week 24 (Table 1). Data was pooled for RTX-US and RTX-EU for efficacy assessments. A similar proportion of patients were retreated in both the groups (GP2013: 70% and RTX: 74%). PD responses remained similar up to the end of the observation period at week 52 (Figure 1). Change from baseline in DAS28 (CRP) at week 24 was -2.07 (Standard error [SE] =0.108) and -2.11 (SE=0.095) in the GP2013 and the RTX groups, respectively. The difference of 0.04 (95% CI: -0.241, 0.323) was below the pre-defined non-inferiority margin of 0.6. ACR20 response rate was 72.3% (95% CI: 64.2%, 80.3%) and 67.3% (95% CI: 59.9%, 74.7%) in the GP2013 and RTX groups at week 24, respectively. Efficacy outcome remained similar up to week 52 (Figure 2). The rates of adverse events were similar between the groups. Anti-drug antibodies (ADAs) were detected in 16.5% of GP2013 and in 15.1% of RTX-treated patients up to last visit. The ADAs were transient in the majority of patients and neutralizing ADAs were detected in 5 and 1 patient in GP2013 and RTX group, respectively.
Conclusion: The study met its primary objective by demonstrating 3-way PK bioequivalence of GP2013, RTX-EU and RTX-US. Three-way PD equivalence, as measured by the depletion of peripheral B cells was also demonstrated. Furthermore, GP2013 and RTX were similar in terms of efficacy, safety and immunogenicity up to week 52.
To cite this abstract in AMA style:
Smolen JS, Cohen SB, Scheinberg M, Shisha T, Kollins D, Zhu P, Cen L, Kivitz AJ, Balanescu AR, Gomez-Reino JJ, Tony HP. A Randomized, Double Blind Trial over 52 Weeks to Demonstrate Bioequivalence of GP2013 and Reference Rituximab in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/a-randomized-double-blind-trial-over-52-weeks-to-demonstrate-bioequivalence-of-gp2013-and-reference-rituximab-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-double-blind-trial-over-52-weeks-to-demonstrate-bioequivalence-of-gp2013-and-reference-rituximab-in-patients-with-rheumatoid-arthritis/