Session Information
The 2020 Pediatric Rheumatology Symposium, originally scheduled for April 29 – May 2, was postponed due to COVID-19; therefore, abstracts were not presented as scheduled.
Session Type: Poster Breakout Session
Session Time: 4:30PM-5:00PM
Background/Purpose: Adult-onset Still’s disease (AOSD) and systemic juvenile idiopathic arthritis (SJIA) are rare systemic disorders of auto-inflammatory nature. There is a growing understanding that SJIA and AOSD are one disease representing an age continuum of the same condition, i.e. Still’s disease. Still’s disease is associated with an activated IL-1 pathway. The anaSTILLs study (anakinra in Still´s disease) was designed to further evaluate efficacy and safety of anakinra in patients with Still´s disease across all age groups.
Methods: The anaSTILLs study was a randomized, double-blind, placebo-controlled, 12-week study including patients with active and newly diagnosed Still´s disease (within 6 months) according to adapted ILAR criteria if < 16 years of age or Yamaguchi criteria if ≥16 years of age at disease onset. Patients were randomized to anakinra 2 mg/kg (max 100 mg/day), 4 mg/kg (max 200 mg/day) or placebo. The primary objective was to demonstrate efficacy of anakinra versus placebo as assessed by ACR30 response with absence of fever at Week 2. Secondary objectives included: early onset of efficacy, sustained efficacy, time to study drug discontinuation, safety, pharmacokinetics (PK), clinical signs and biomarkers.
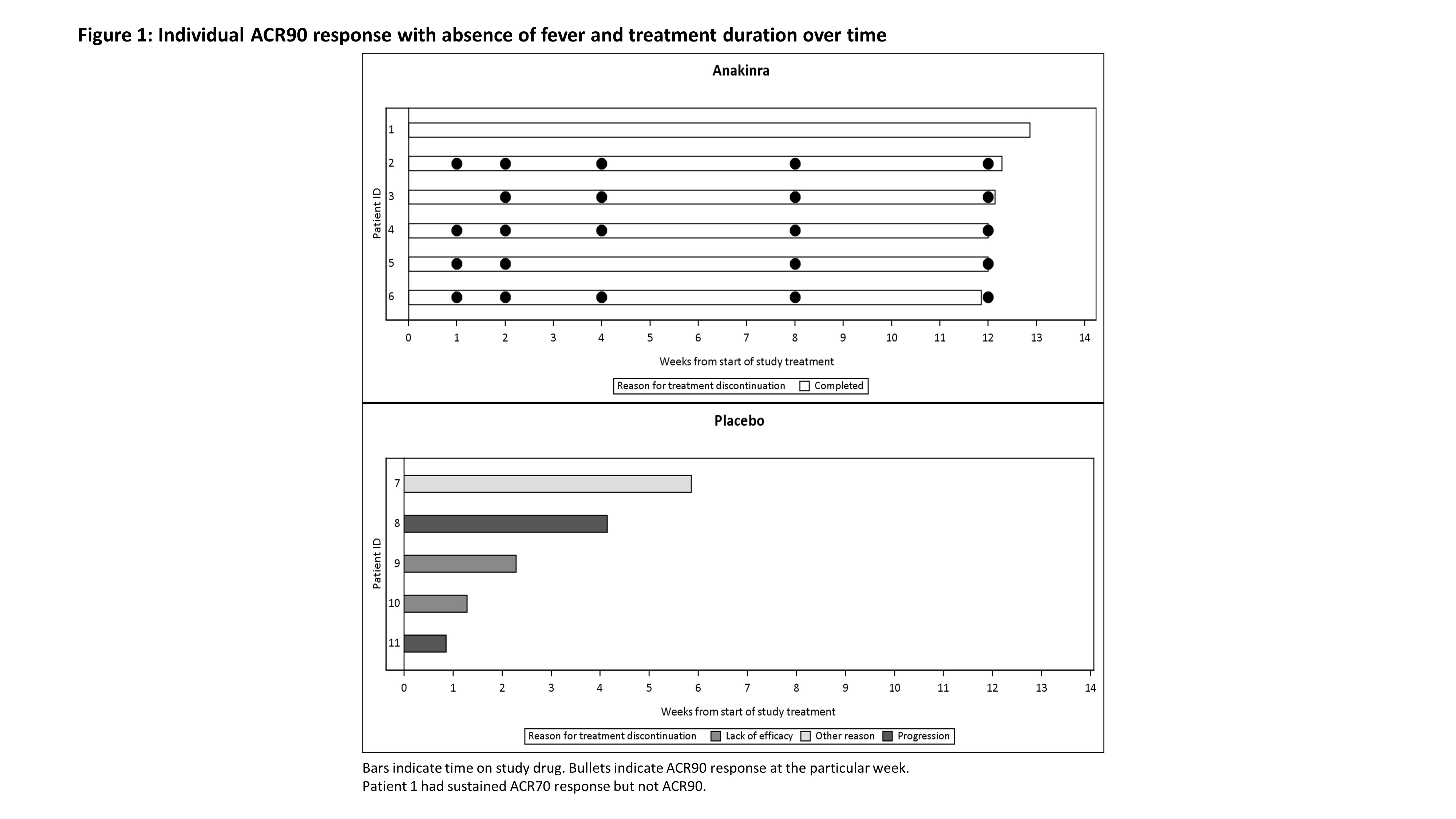
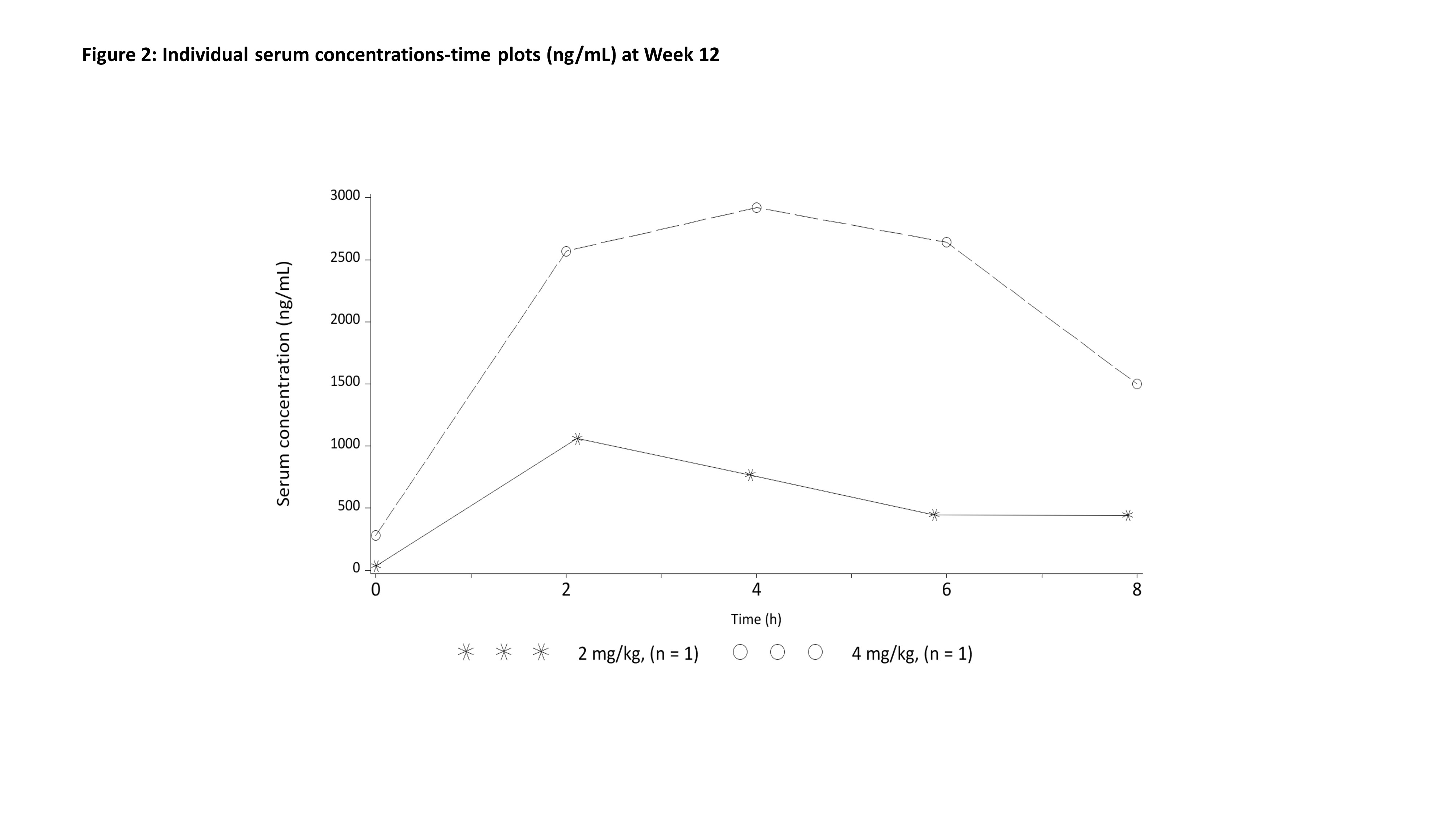
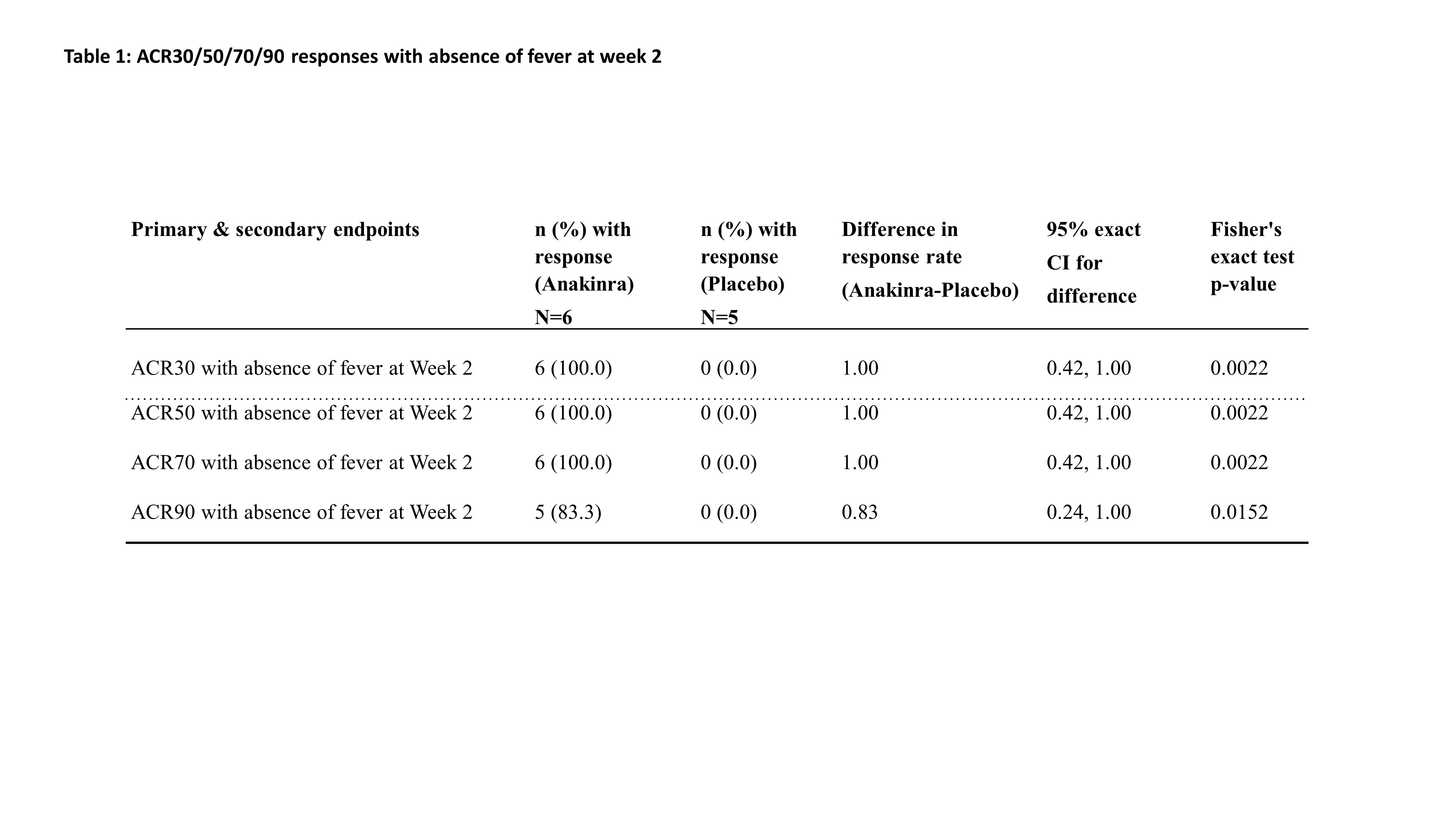
Results: 12 patients were randomized and received study treatment: 6 to anakinra (2 mg/kg n=2, 4 mg/kg n=4) and 6 to placebo (the study was discontinued early due to slow recruitment). One patient in the placebo group had lymphoma, not Still’s disease, and was excluded from efficacy analyses. 11 patients were analyzed for efficacy (anakinra n=6, placebo n=5), 8 were children [median (range) age (years)=4.0 (1-11)] and 3 were adults [median (range) age (years)=32.0 (25-51)]. 55% were male and the mean symptom duration was 74.2 days. All patients on anakinra but none on placebo achieved ACR30 response with absence of fever at Week 2 (p-value=0.0022). The efficacy of anakinra was supported by superiority to placebo in ACR50/70/90 responses with absence of fever at Week 2. All placebo patients discontinued the study within 6 weeks, 2 due to progressive disease, 2 due to lack of efficacy and 1 due to withdrawal by patient. Early onset of efficacy at Week 1 was observed by a numerically higher proportion of ACR responders in the anakinra group compared to placebo. ACR30/50/70/90 responses in anakinra-treated patients were sustained throughout the study period. No unexpected safety findings were observed. Serum concentrations and PK parameters of anakinra were determined at Week 12 in 2 pediatric patients. In a 1-year-old (4.1 mg/kg) and a 6-year-old (1.5 mg/kg) patient, Cmax of 2920 ng/mL and 1060 ng/ml, was reached 4.0 and 2.1 hours after administration of anakinra, respectively. CL/F and Vd/F were 139 mL/h/kg and 1187 mL/kg, and 203 mL/h/kg and 1322 mL/kg, respectively.
Conclusion: Anakinra is superior to placebo in the treatment of Still’s disease. The safety data and steady-state PK parameters are consistent with previous anakinra experience. These results confirm the benefits of anakinra treatment in patients with active, newly diagnosed Still´s disease across ages.
To cite this abstract in AMA style:
Schanberg L, Nigrovic P, Cooper A, Chatham W, Akoghlanian S, Singh N, Rabinovich C, Thatayatikom A, Taxter A, Hausmann J, Zdravkovic M, Ohlman S, Andersson H, Cederholm S, Huledal G, Schneider R, De Benedetti F. A Randomized, Double-blind, Placebo-controlled Study of Anakinra in Pediatric and Adult Patients with Still’s Disease [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 4). https://acrabstracts.org/abstract/a-randomized-double-blind-placebo-controlled-study-of-anakinra-in-pediatric-and-adult-patients-with-stills-disease/. Accessed .« Back to 2020 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-double-blind-placebo-controlled-study-of-anakinra-in-pediatric-and-adult-patients-with-stills-disease/