Session Information
Date: Monday, November 9, 2020
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster III: Therapies
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Still’s disease, including both systemic juvenile idiopathic arthritis (SJIA) and adult-onset Still’s disease (AOSD), is a rare systemic auto-inflammatory disorder associated with an activated IL-1 pathway. The purpose of the anaSTILLs study (anakinra in Still’s disease) was to build on earlier evidence and evaluate, in a controlled setting, the efficacy and safety of anakinra (IL-1 receptor antagonist), in patients with active, newly diagnosed Still’s disease across all age groups.
Methods: The anaSTILLs study (NCT03265132) was a randomized, double-blind, placebo-controlled, 12-week study including patients with active and newly diagnosed Still’s disease (adapted ILAR criteria if < 16 years of age, Yamaguchi criteria if ≥16 years of age at disease onset). Patients were randomized to anakinra 2 mg/kg (max 100 mg/day), 4 mg/kg (max 200 mg/day) or placebo. The primary objective was to demonstrate the efficacy of anakinra versus placebo as assessed by ACR30 response with absence of fever at Week 2. Secondary objectives included: early onset of efficacy, sustained efficacy, time to study drug discontinuation, safety, pharmacokinetics (PK), clinical signs and biomarkers.
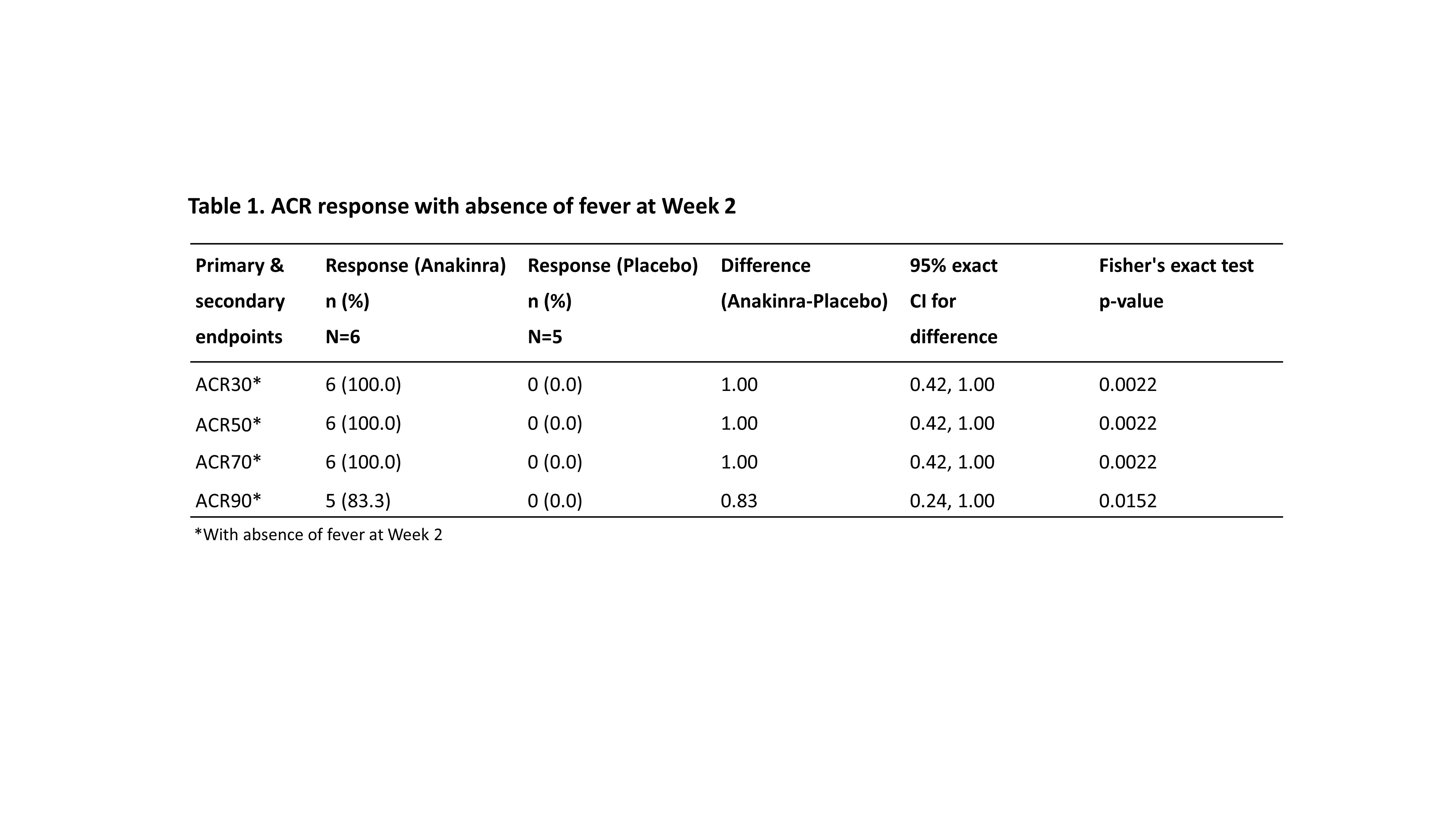
Results: Twelve patients were randomized and received study treatment: 6 to anakinra (2 mg/kg n=2, 4 mg/kg n=4) and 6 to placebo. Due to slow recruitment the study was terminated early. One patient was excluded from the efficacy analyses since he/she was later diagnosed with lymphoma, not Still’s disease. 11 patients were analyzed for efficacy (anakinra n=6, placebo n=5), 8 children (median [range] age=4.0 [1-11] years) and 3 adults (median [range] age=32.0 [25-51] years). 55% were male and the mean (range) symptom duration was 74.2 (12-222) days. All patients on anakinra but none on placebo achieved ACR30 response with absence of fever at Week 2 (p-value=0.0022). The efficacy of anakinra was supported by superiority to placebo in ACR50/70/90 responses with absence of fever at Week 2. All placebo patients discontinued the study within 6 weeks, 2 due to progressive disease, 2 due to lack of efficacy and 1 due to withdrawal by the patient. A numerically greater proportion of patients in the anakinra group had early onset of efficacy (Week 1) compared to placebo, as assessed by ACR30. ACR30/50/70/90 responses in anakinra-treated patients were sustained throughout the study period. No unexpected safety findings were observed. Based on serum anakinra concentrations Week 12, Cmax was 2920 ng/mL at 4.0 hours (dose: 4.1 mg/kg) in a 1-year-old patient, and Cmax was 1060 ng/mL at 2.1 hours (dose: 1.5 mg/kg) in a 6-year-old patient.
Conclusion: Anakinra is superior to placebo in the treatment of Still’s disease. The safety and PK results are consistent with previous anakinra experience. These results confirm the benefits of anakinra treatment in patients with active, newly diagnosed Still’s disease across ages.
To cite this abstract in AMA style:
Schanberg L, Nigrovic P, Cooper A, Chatham W, Akoghlanian S, Singh N, Rabinovich C, Thatayatikom A, Taxter A, Hausmann J, Zdravkovic M, Ohlman S, Andersson H, Cederholm S, Huledal G, Schneider R, De Benedetti F. A Randomized, Double-Blind, Placebo-Controlled Study of Anakinra in Pediatric and Adult Patients with Still’s Disease [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/a-randomized-double-blind-placebo-controlled-study-of-anakinra-in-pediatric-and-adult-patients-with-stills-disease-2/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-double-blind-placebo-controlled-study-of-anakinra-in-pediatric-and-adult-patients-with-stills-disease-2/