Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: 30+ million US adults have OAKP. After years of using NSAIDs and other conservative measures many patients remain with OAKP. CNTX is a 1 mg dose of capsaicin for IAI to reduce OAKP.
Methods: This was a randomized, double-blind, placebo-controlled, 52-week study with 325 subjects dosed, to evaluate the efficacy and safety of a single IAI (at Day 1) of 1.0 mg of CNTX, compared with placebo, in subjects (both sexes) with chronic, moderate-to-severe index OAKP (MSIOAKP). Subjects were allowed specific OAKP concomitant and rescue medications during the study.
Subjects with MSIOAKP using a numeric pain rating scale between 5-9 (NPRS [0 – 10; 0 = no pain, 10 worst pain possible]), Kellgren-Lawrence (KL) grades 2-4 (0-4, Normal to severe by radiographs), and met knee OA (KOA) diagnostic criteria, were blindly randomized on Day 1 to a single IAI of capsaicin or placebo (3:2) into the most painful knee (index). NPRS at Week 12 was the primary outcome measure, with supporting data from the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC; A=pain, B= knee stiffness, C= function) and Patient Global Impression of Change (PGIC; 0-7 scale). Radiographic data was taken of both knees.
Key safety included physical exams, vital signs, ECGs, labs, and fixed flexion radiographs of both knees at Screening, Weeks 12 and 52.
Results: 325 subjects received 1 dose of study drug (136 placebo, 189 CNTX); 262 randomized subjects completed; 70 subjects discontinued (DC) early. Reported reasons for study DC were subject withdrawal (7.2%), lost to follow-up (5.1%), and other (2.1%). 15 (4.5%) subjects DC prior to Week 12. Subjects: Mean age 61.8 years (range = 40-85 years), 63.4% female, 81.5% -not Hispanic or Latino; 65.5% Caucasian and 23.4% Back/African American.
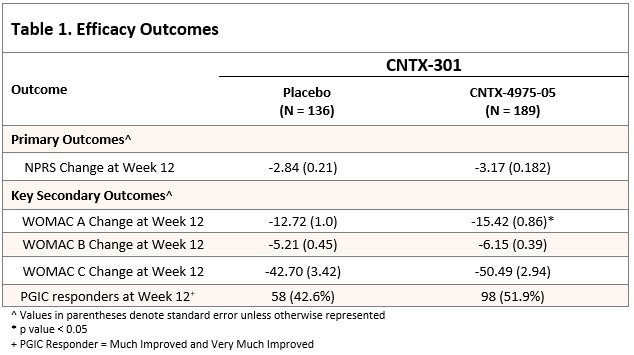
Mean index knee baseline NPRS was 7.07, WOMAC A (0-50 scale; 0 = severe pain) 31.45, WOMAC B (0-20 scale) and WOMAC C (0-170) 13.26 and 106.99, respectively.
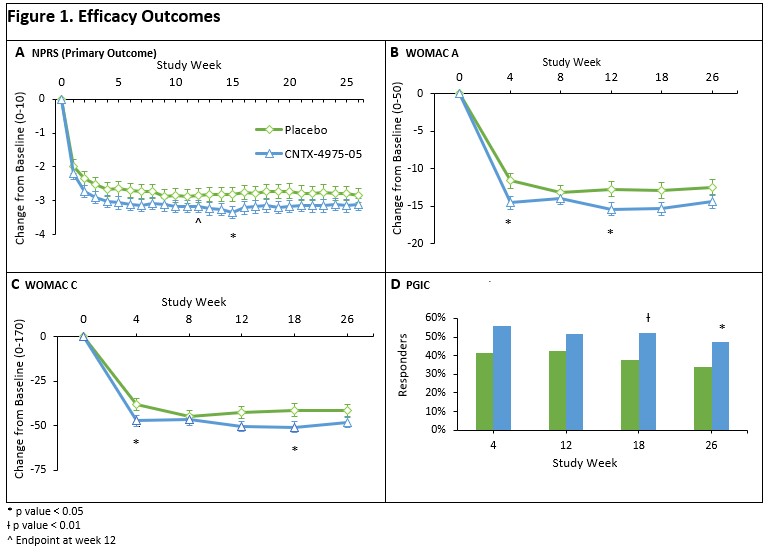
The least squares (LS) mean reduction in the average weekly pain with walking NPRS (0-10) score for the index knee was greater in the CNTX group than in the placebo group (−3.17 vs −2.84, respectively; p = 0.1904). However, the study failed to meet the primary efficacy endpoint. The WOMAC A, B and C subscales, the mean changes from study baseline were numerically better at nearly all visits through Week 26, and usually better to Week 52 for CNTX relative to placebo. Further, a greater proportion of subjects in the CNTX group were considered PGIC responders at Week 12 and beyond -(Table 1 and Figure 1).WOMAC A (pain) for CNTX was significantly (p< 0.05) improved over placebo at week 12. Overall safety was acceptable and CNTX after treatment Day 1, where there was more transient post injection pain in the CNTX group. Knee radiographs (masked central reading) from Baseline to Week 52 showed no difference in KOA progression between placebo and CNTX, with no rapidly progressive osteoarthritis.
Conclusion: The study did not meet the primary endpoint, though analgesic efficacy through Week 26 in CNTX was numerically superior to placebo. Improvement in WOMAC A at 12 weeks was significantly greater for CNTX compared to placebo CNTX safety was acceptable.
To cite this abstract in AMA style:
Stevens R, Campbell J, Newman C, Connolly J. A Randomized, Double-blind, Placebo-controlled, Single Injection, 52-Week Study to Evaluate the Efficacy and Safety of an Intra-articular Injection (IAI) of CNTX-4975-05 (CNTX) in Subjects with Chronic, Moderate-to-severe Osteoarthritis Knee Pain (OAKP) – Study 301 [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/a-randomized-double-blind-placebo-controlled-single-injection-52-week-study-to-evaluate-the-efficacy-and-safety-of-an-intra-articular-injection-iai-of-cntx-4975-05-cntx-in-subjects-with-chroni/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-double-blind-placebo-controlled-single-injection-52-week-study-to-evaluate-the-efficacy-and-safety-of-an-intra-articular-injection-iai-of-cntx-4975-05-cntx-in-subjects-with-chroni/