Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Induction of remission for severe ANCA-associated vasculitis [granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA)] utilizes rituximab (RTX) or cyclophosphamide (CYC) and tapering doses of glucocorticoids (GC), followed by either a second course of RTX or other immunosuppressive for remission maintenance. There is a strong need for therapies that either replace or markedly decrease the doses of GC used to treat AAV. Vilobelimab (IFX-1) is a monoclonal antibody that specifically binds to the soluble human complement fragment C5a. In pre-clinical studies, vilobelimab was found to bind C5a rapidly and result in near complete blockade of C5a-induced biological effects while not affecting cleavage of C5 or formation of the membrane attack complex.
Methods: The IXCHANGE Trial (NCT03895801), a prospective, randomized, double-blind, double-dummy, active-controlled, 2-part Phase II study, evaluated the efficacy of vilobelimab as a replacement for GC in patients with GPA and MPA. Part 1 of the study compared vilobelimab + reduced-dose GC (RDGC) to standard-dose GC (SDGC) as add-on to standard of care RTX or CYC, while part 2 compared vilobelimab alone to SDGC in the presence of RTX or CYC. After loading doses at 1, 4, and 8 days, patients were given 800 mg vilobelimab every 2 weeks for 16 weeks followed by an 8-week observation period. Primary efficacy evaluated the proportion of subjects achieving clinical response [reduction in Birmingham Vasculitis Activity Score (BVAS) of ≥50% compared to baseline and no worsening in any body system to week 16]. Non-responders were those who received rescue medication prior to the assessment time point or discontinued due to a related adverse event (AE), lack of efficacy, or progressive disease. Clinical remission (BVAS=0) was assessed at 4, 8, 12, and 16 weeks. Secondary endpoints included safety, estimated glomerular filtration rate (eGFR), and the glucocorticoid toxicity index (GTI).
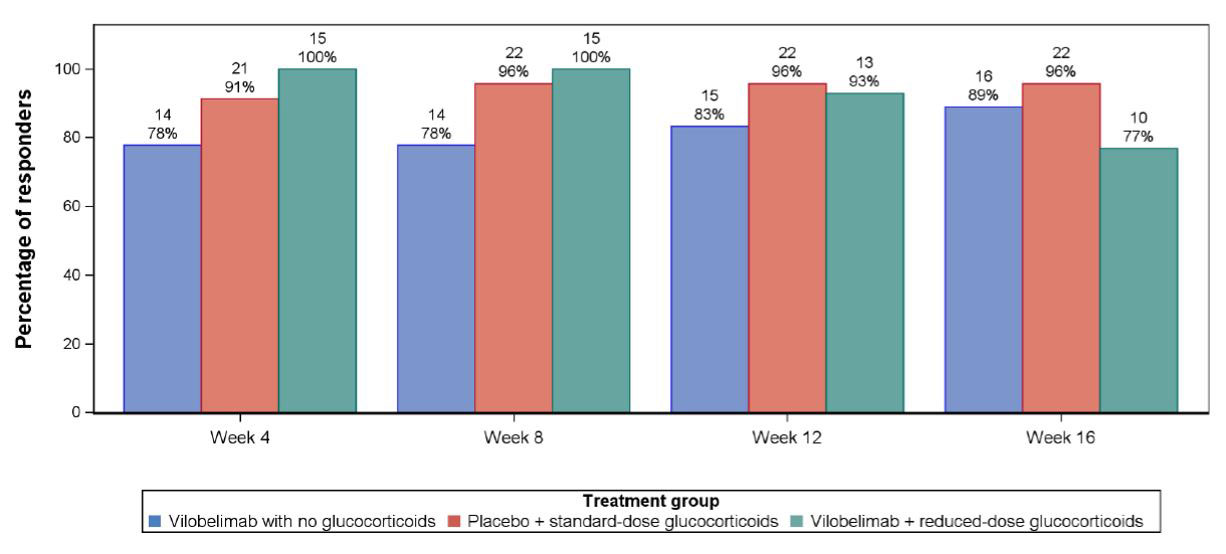
Results: Fifty-one of 57 subjects randomized completed the study. The cumulative mean GC dose received 28 days prior to randomization was comparable in all arms. The mean total cumulative dose of GC from Day 1 (1st dose of IMP) until end of study was 541.9 mg for vilobelimab alone, 3751.3 mg for SDGC, and 1485.8 mg for vilobelimab + RDGC. Clinical response (Figure 1) and clinical remission rates were similar across the three arms at week 16: response: 89% vs 96% vs 77%; remission: 78% vs 87% vs 77%. The mean (SD) for GTI at week 16 was substantially lower with vilobelimab alone [0.8 (9.0)] when compared to the means for SDGC [44.9 (41.5)] and vilobelimab + RDGC [26.1 (39.2)]. There were no medically meaningful changes in eGFR in any arm. The vilobelimab-only group had the lowest number of reported treatment emergent AE (TEAE). In total, there were 14 serious TEAE (5, vilobelimab alone; 6, SDGC; and 3, vilobelimab + RDGC).
Conclusion: Though the IXCHANGE Trial was not powered to demonstrate non-inferiority of vilobelimab alone compared to SDGC, results from the trial suggest that treatment of GPA and MPA with vilobelimab has the potential to induce remission together with RTX and CYC with substantial reduction in the dose and associated toxicity of GC.
To cite this abstract in AMA style:
Merkel P, Hellmich B, Pfaff A, Müller C, Startseva E, Jayne D. A Randomized, Double-Blind, Phase II Study of Glucocorticoid Replacement by Vilobelimab, an Anti-C5a Monoclonal Antibody, in ANCA-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/a-randomized-double-blind-phase-ii-study-of-glucocorticoid-replacement-by-vilobelimab-an-anti-c5a-monoclonal-antibody-in-anca-associated-vasculitis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-double-blind-phase-ii-study-of-glucocorticoid-replacement-by-vilobelimab-an-anti-c5a-monoclonal-antibody-in-anca-associated-vasculitis/