Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: To evaluate the long-term safety, immunogenicity (IG), and efficacy of the adalimumab (ADL) biosimilar, PF-06410293 (ADL-PF), in patients (pts) with moderate to severe active RA who continued ADL-PF treatment throughout 78 weeks (wks) or who switched from reference ADL sourced from the European Union (ADL-EU) to ADL-PF at wk 26 or wk 52 in a randomized, double-blind, comparative clinical trial (NCT02480153). This report includes results from wks 52-92 (including 16-wk follow-up [FU]).
Methods: Eligible pts met 2010 ACR/EULAR RA diagnosis criteria for ≥4 months, had an inadequate response to MTX, and had received ≤2 doses of 1 lymphocyte-depleting or non-ADL biologic. Pts stratified by geographic regions were initially randomized (1:1) in treatment period 1 (TP1) to ADL-PF or ADL-EU (40 mg subcutaneously every other wk), both with MTX (10–25 mg/wk). The primary endpoint was ≥20% clinical improvement of ACR criteria (ACR20) at wk 12. Secondary efficacy endpoints included ACR20 other than at wk 12, DAS28; 4 components based on high-sensitivity CRP (DAS28-4[CRP]), and other measures of clinical response or remission. At wk 26 (start of TP2), pts receiving ADL-EU were blindly re-randomized (1:1) to remain on ADL-EU or transition to ADL-PF for 26 wks. At wk 52 (start of TP3), all pts received open-label treatment with ADL-PF for 26 wks; 3 groups (gps) were evaluated corresponding to the treatment sequence during the study: biosimilar; wk 26 switch; wk 52 switch.
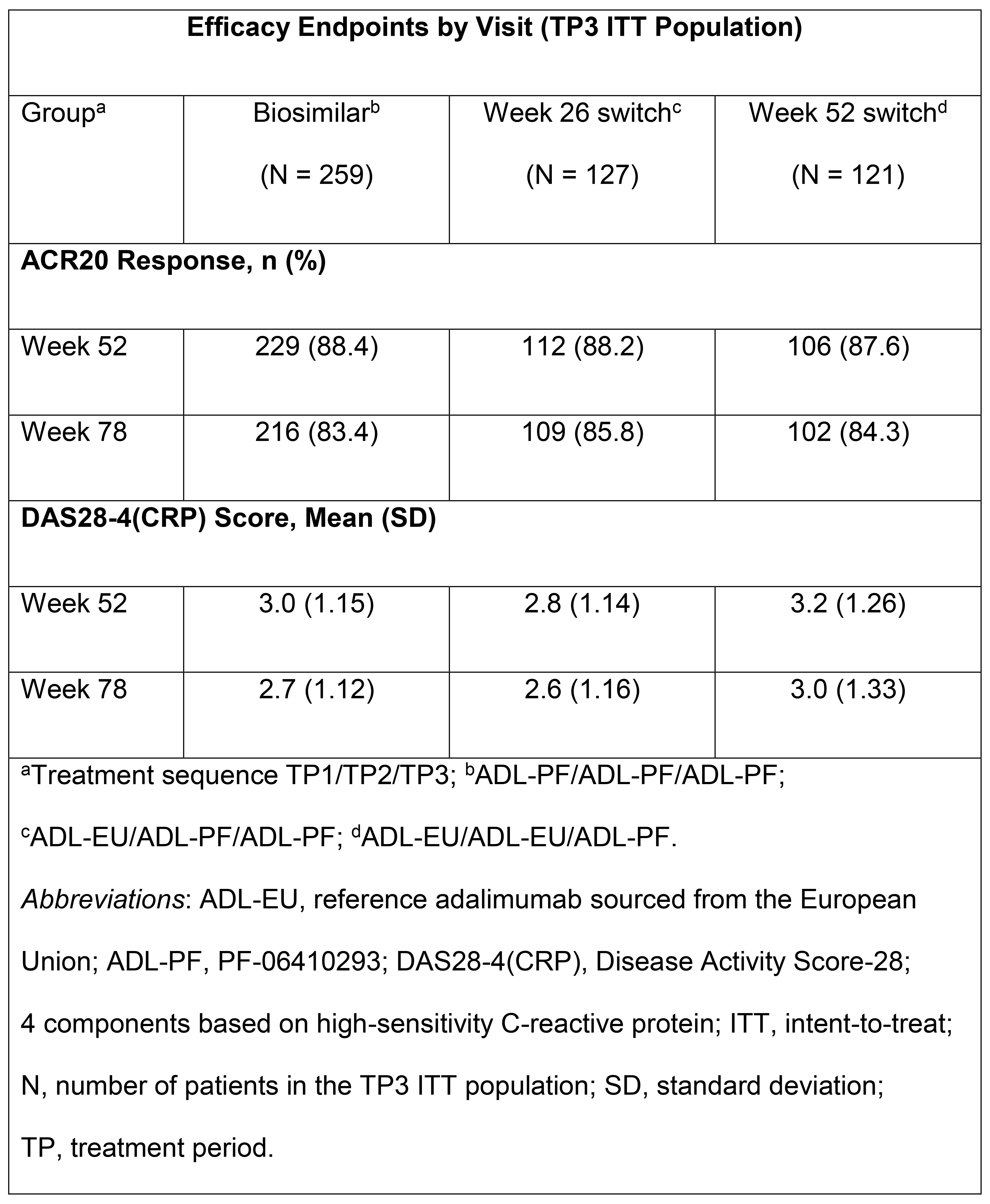
Results: Of the randomized TP1 pts (N=597), 552 pts entered TP2. At wk 52, all pts remaining on ADL-EU were switched to ADL-PF; 507 pts continued to participate in TP3. The majority of TP3 pts were female (78.1%) and White (86.6%). ACR20 response rates and DAS28-4(CRP) scores were sustained and comparable across gps (Table). ADL-PF was generally well tolerated, with a comparable safety profile across gps. Incidence of treatment-emergent adverse events (AEs) during TP3 and FU was 42.6% (biosimilar), 37.0% (wk 26 switch), and 50.8% (wk 52 switch); 3 (0.6%) pts overall (all in the wk 52 switch gp) reported treatment-related serious AEs. There was 1 death (wk 52 switch gp). Pre-dose (TP3) anti-drug antibody (ADA) prevalence at wk 52 was 41.5%, 42.5%, and 48.3% for the biosimilar, wk 26, and wk 52 switch gps, respectively. The incidences of pts with a first positive ADA result during TP3 or FU, among pts who were ADA negative on entry to TP3, were 8.9%, 6.3%, and 8.3% for the biosimilar, wk 26, and wk 52 switch gps, respectively; overall, incidences of pts with ADAs in TP3 and FU were comparable among gps (46.1%, 46.5%, and 54.2%, respectively).
Conclusion: Results from TP3 and FU (wks 52–92) are consistent with earlier findings from this study, demonstrating no clinically meaningful differences in safety, IG, and efficacy between treatment gps, independent of a single treatment switch from ADL-EU to ADL-PF at wk 26 or wk 52.
To cite this abstract in AMA style:
Fleischmann R, Alvarez D, Bock A, Cronenberger C, Vranic I, Zhang W, Alten R. A Randomized, Double-blind Phase 3 Study Comparing the Efficacy, Safety and Immunogenicity of PF-06410293 (Abrilada™), an Adalimumab (ADL) Biosimilar, and Reference ADL (Humira®) in Patients with Moderate to Severe Active RA: Results from Weeks 52-92 [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/a-randomized-double-blind-phase-3-study-comparing-the-efficacy-safety-and-immunogenicity-of-pf-06410293-abrilada-an-adalimumab-adl-biosimilar-and-reference-adl-humira-in-patie/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-double-blind-phase-3-study-comparing-the-efficacy-safety-and-immunogenicity-of-pf-06410293-abrilada-an-adalimumab-adl-biosimilar-and-reference-adl-humira-in-patie/