Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Pharmacological therapies are limited, associated with off-target effects, are frequently contraindicated, and only modestly effective for pain in osteoarthritis (OA). Effusion and synovitis are common in OA and are associated with symptomatic and structural progression of OA. Curcuma longa (Turmeric) extract has anti-inflammatory effects and is gaining popularity in the treatment of OA despite the lack of high-quality evidence. The CurKOA trial aimed to compare the efficacy of Curcuma longa extract versus identical placebo for treating knee pain and effusion-synovitis in an inflammatory phenotype of OA identified using ultrasound scan.
Methods: In this double-blind trial, we randomised 70 participants with significant knee pain ( ≥40 mm knee pain on visual analogue score [VAS] scale), knee OA (ACR clinical criteria) and presence of a moderate amount of ultrasound-defined effusion/synovitis ( ≥4 mm thickness in the suprapatellar region) to receive Curcuma longa extract (80% Turmerosaccharides and 20% Curcumin extract, 2×500 mg capsules/day) (n=36) or identical placebo (n=34) for 12 weeks. The primary outcome measures were change in knee pain (assessed by VAS) and MRI-defined effusion-synovitis volume over 12 weeks. Secondary outcomes included changes in knee pain and function (WOMAC), OARSI-OMERACT treatment responders and change in T2 relaxation time (milliseconds, ms) of the cartilage. Linear mixed-effects models were used, with adjustment for baseline values of the outcome measure.
Results: Of the 112 participants screened, a total of 70 participants (age 61.8±8.6 years, 56% female) were randomised, and 68 (97%) completed the 12 week assessments.
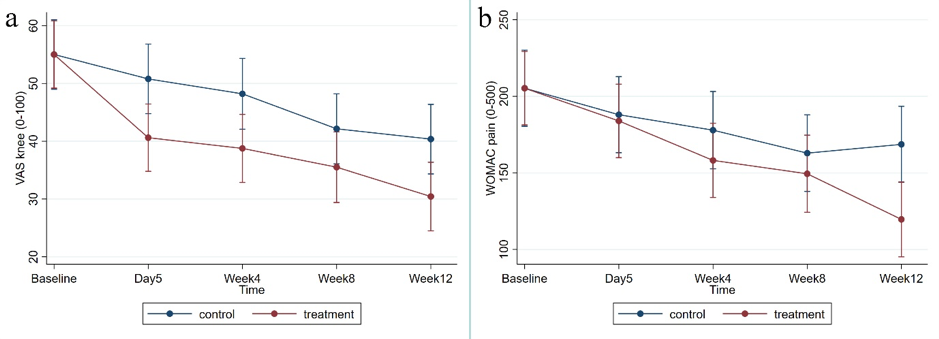
Primary outcomes: There was a reduction in VAS knee pain in the treatment (-23.75 [-29.78 to -17.73]) and placebo (-14.64 [-20.80 to -8.47]) group, with a significant between-group difference of -9.11mm [-17.79 to -0.44] (Figure 1a) equivalent to a standard effect size of 0.49. There was a reduction in the MRI assessed effusion-synovitis volume in the treatment (-2.38 mL [-4.09 to -0.68]) and placebo (-3.63 mL [-5.39 to -1.88]) group, with no significant between-group difference (1.25 mL [-1.21 to 3.72]).
Secondary outcomes: Significant changes favouring the treatment group were seen in WOMAC knee pain (between-group difference: -47.22 [-81.22 to -13.22]) (Figure 1b), WOMAC function (between-group difference: -112.26 [-222.79 to -1.74]) and OARSI-OMERACT treatment responders (63% in the treatment group and 38% in the placebo group [Risk Ratio, RR=1.64 (1.00 to 2.70)]. No significant change in T2 relaxation time of the femoral cartilage (average values of T2 relaxation time between group difference -0.38 ms [-1.10 to 0.34]) was seen. The number of adverse events was similar in the treatment (n=18) and placebo (n=29) groups, and there were no adverse events related to the treatment.
Conclusion: Curcuma longa extract significantly improved knee pain in an inflammatory phenotype of knee OA patients over 12 weeks. There was a moderate standard effect size of the treatment which appears greater than other conventional pharmacological therapies. In this short term study, Curcuma longa extract had no effect on knee structural measures assessed using MRI.
To cite this abstract in AMA style:
Wang Z, Jones G, Winzenberg T, Cai G, Laslett L, Aitken D, Hopper I, Jones R, Ding C, Fripp J, Antony B. A Randomised Clinical Trial of Curcuma Longa Extract for Treating Symptoms and Effusion-Synovitis of Knee Osteoarthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-randomised-clinical-trial-of-curcuma-longa-extract-for-treating-symptoms-and-effusion-synovitis-of-knee-osteoarthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomised-clinical-trial-of-curcuma-longa-extract-for-treating-symptoms-and-effusion-synovitis-of-knee-osteoarthritis/