Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Mycophenolate Mofetil/Mycophenolic acid (MMF) is an immunosuppressant used to treat SLE. Due to the teratogenic effects of MMF, the FDA recommended universal risk evaluation and mitigations strategies (MREMS) to prevent unplanned pregnancies, minimize fetal exposure and collect information on pregnancy outcomes. MREMS is not widely utilized in clinical practice. We designed a quality improvement initiative to implement MREMS counseling, documentation and pregnancy screening.
Methods: The study took place in an academic lupus clinic from February 2023 to May 2024. MREMS protocol was recommended for all adult female SLE patients age 18-50 of age on MMF seen during in-person visit, excluding those with total hysterectomy. We educated providers and nurses on the protocol which included: 1) Required encounter note documentation of counseling of teratogenic nature of MMF and need for contraception and 2) Ordering of pregnancy screening test at each visit. We executed multiple plan-do-study-act cycles that included: creating an algorithm for nursing to identify eligible females and order pregnancy tests, modifying electronic record templates, provider and nurse feedback. We also had quarterly QI team feedback sessions. We continued cycles until we reached our goal of 75% of eligible encounters meeting protocol.
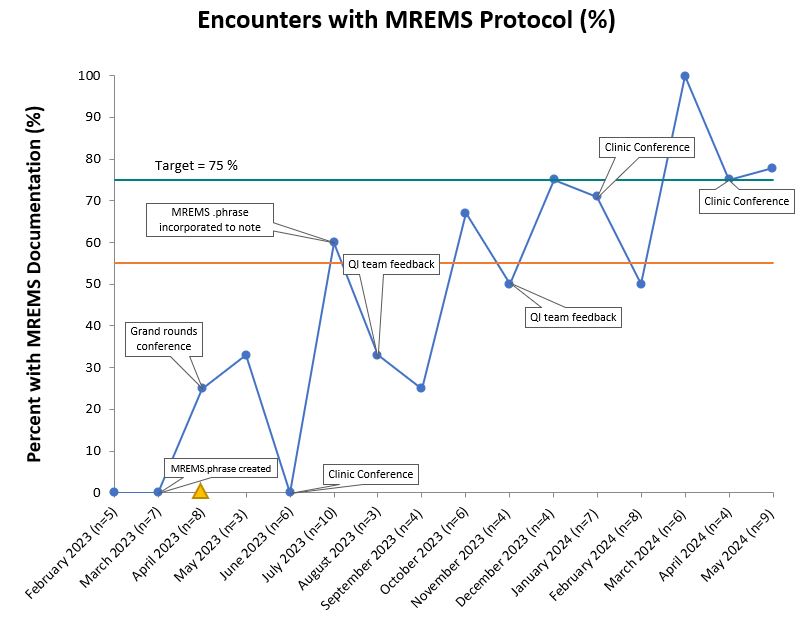
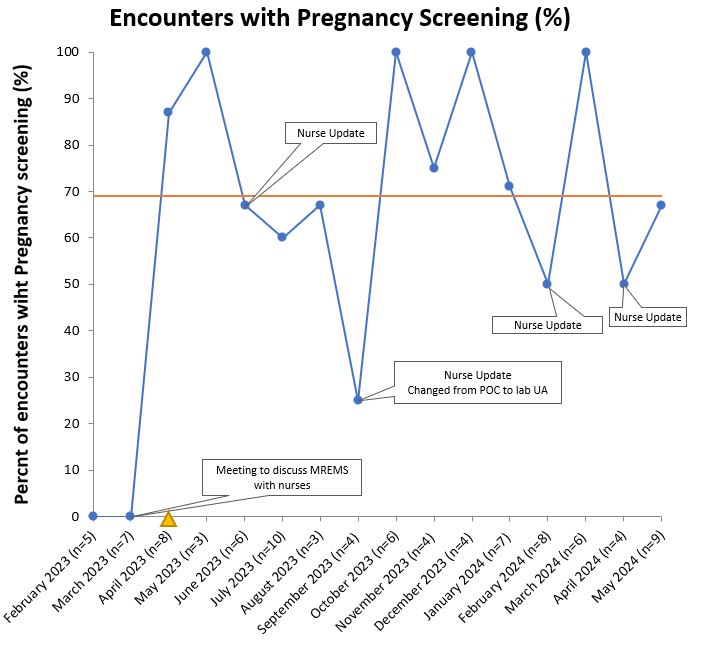
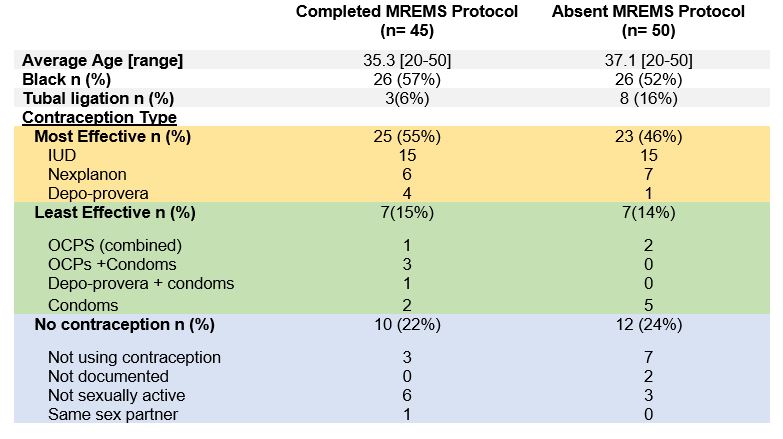
Results: Among eligible encounters (n=95), MREMS documentation increased from 0% to a median of 55% following process improvements generated through PDSA cycles. (Figure 1) This was sustained over 1 year and reached the goal of 75% for three consecutive months. Contraception was documented at 98% of visits. In parallel, pregnancy test screening ordered by nursing staff and physicians also increased from 0% to a median of 69% of encounters. (Figure 2) Over the course of the intervention we did not have any pregnancies while on MMF. Among the 62 participants on contraception, 48% (n=30) were using highly effective birth control. (Table 1) Among encounters with absent MREMS, more women had tubal ligation when compared to completed MREMS encounters (16% vs 8%). We did not appreciate any differences in age or race among those with absent MREMS documentation. Implementation challenges given by providers included justifying pregnancy test costs and lower relevance of MREMS protocol in patients on effective long-term contraception, same sex partners or tubal ligation.
Conclusion: Using quality improvement cycles, we incorporated a two-part MREMS protocol into a busy academic lupus clinic, with improved documentation and pregnancy screening. We did not have any unplanned pregnancies, nearly all patients had documented contraception, and half were on recommended birth control. Future efforts will focus on improving counseling and pregnancy screening for women not on contraception.
To cite this abstract in AMA style:
Cintron D, Rogers J, Sadun R, Maheswaranathan M, Sun K, Doss J, Criscione-Schreiber L, Clowse M. A Qualitative Improvement Project to Incorporate the Mycophenolate Risk Evaluation and Mitigation Strategies in an Academic Lupus Clinic [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-qualitative-improvement-project-to-incorporate-the-mycophenolate-risk-evaluation-and-mitigation-strategies-in-an-academic-lupus-clinic/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-qualitative-improvement-project-to-incorporate-the-mycophenolate-risk-evaluation-and-mitigation-strategies-in-an-academic-lupus-clinic/