Session Information
Date: Wednesday, October 29, 2025
Title: Abstracts: Systemic Lupus Erythematosus – Treatment II (2693–2698)
Session Type: Abstract Session
Session Time: 12:30PM-12:45PM
Background/Purpose: Tacrolimus (TAC) and glucocorticoid as continuous induction-maintenance therapy for lupus nephritis (LN) has never been investigated. We conducted a prospective randomized open-label multicenter study to evaluate its efficacy and safety against standard-of-care mycophenolate mofetil (MMF) and glucocorticoid dual immunosuppression in Asian patients with active severe LN.
Methods: Patients with biopsy-proven Class III/IV±V LN were randomized to receive pulse methylprednisolone (500mg/500mg/150mg) for 3 days followed by oral prednisolone (0.7 mg/kg/day, then taper) in combination with either TAC (initial dose 2 mg bid; target trough level 6-8 ng/mL) or MMF (1 g bid), then followed for 96 weeks. Primary endpoint was sustained renal response (SRR) [defined as proteinuria reduced by >50% compared with baseline and 24-hr urine protein < 1 g and serum creatinine not higher than 15% above baseline and no disease flare or rescue therapies] at Week 96. Key secondary endpoints included rates of complete renal response (CR), adverse events, and time-to-renal related adverse events/death.
Results: A total of 130 patients were randomized (65 in each group). TAC and MMF treatment groups showed similar rates of SRR (58.5% vs. 69.2%, OR 0.62; 95% CI 0.29-1.29; p=0.2) and CR (55.4% vs. 63.1%, OR 0.72; p=0.36) at Week 96. SRR rates for TAC and MMF groups were 53.8% vs 52.3% at Week 24, and 63.1% vs. 66.2% at Week 48 respectively (Figure 1). Time-to-renal related adverse events/death were similar in both groups (HR 0.70; 95% CI 0.37-1.35; p=0.29). Overall adverse event rates were 69.2% and 70.8% in TAC and MMF groups respectively (p=1.00), while serious adverse events were more frequent in the MMF group (12.3% vs. 30.8%, p=0.02). Acute kidney injury (16.9% vs. 3.1%) and tremor (16.9% vs. 0%) were more frequent in TAC-treated patients, while leucopoenia (1.5% vs. 12.3%) was more frequent in the MMF group. Three patients in the MMF group died.
Conclusion: Immunosuppressive regimen with TAC combined with glucocorticoid showed comparable efficacy as MMF with glucocorticoid, as continuous induction-maintenance treatment for active LN over 96 weeks, with similar overall adverse event rate but different safety profile between the two treatments.
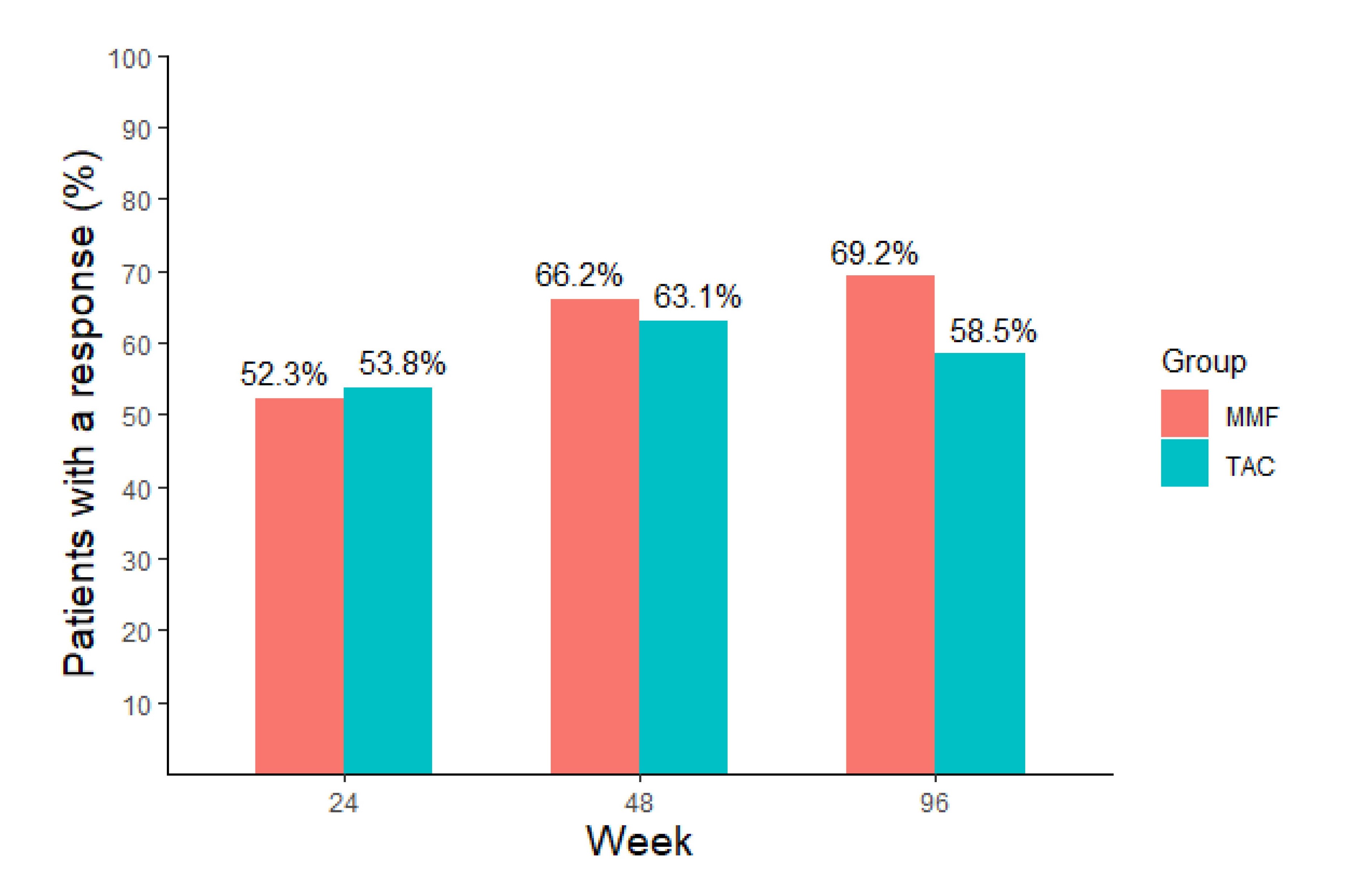 Figure 1. Sustained renal response (SRR) rate in patients with active lupus nephritis treated with tacrolimus (TAC) or mycophenolate mofetil (MMF) in combination with glucocorticoid.
Figure 1. Sustained renal response (SRR) rate in patients with active lupus nephritis treated with tacrolimus (TAC) or mycophenolate mofetil (MMF) in combination with glucocorticoid.
To cite this abstract in AMA style:
Navarra S, Yap D, Ni Z, Avihingsanon Y, Lim S, Jiang N, Chen W, Li C, Zhi H, Zhang C, Chan C, Yung S, Takeuchi T, Chan T. A Prospective Randomized Controlled 96-week Study to Evaluate the Efficacy and Safety of Tacrolimus and Glucocorticoid as Continuous Induction-Maintenance Treatment for Class III/IV±V Lupus Nephritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-prospective-randomized-controlled-96-week-study-to-evaluate-the-efficacy-and-safety-of-tacrolimus-and-glucocorticoid-as-continuous-induction-maintenance-treatment-for-class-iii-iv%c2%b1v-lupus-nephr/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-prospective-randomized-controlled-96-week-study-to-evaluate-the-efficacy-and-safety-of-tacrolimus-and-glucocorticoid-as-continuous-induction-maintenance-treatment-for-class-iii-iv%c2%b1v-lupus-nephr/
