Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Approximately 30-40% of RA patients fail to respond to first biological therapy. Predicting the patient’s responsiveness to the first biological therapy is still an unmet need in the clinical setting. In the case of non-responder (NR) patients it leads to unnecessary exposure, delay of adequate therapy, disease progression and therapy cost increase. By identifying those bio-naive RA patients who are likely responders (Rs) or NRs to infliximab (both intravenous and subcutaneous) treatment, a predictive in vitro testing would have a significant effect on the administration of TNF-α inhibitor therapy, on the real life implementation of effective personalized therapy.
The purpose of this in vitro diagnostic medical device performance evaluation study was to demonstrate that – gene expression profiles of a selected gene set as genomic biomarkers (i.e. the IVD medical device) and a proprietary algorithm for data analysis – predict month 6 therapeutic response to infliximab, discriminate between Rs and NRs. Rs were defined if they reached DAS target value DAS28≤3.2 at 6 month (M6).
Methods: Two hundred twenty bio-naive RA patients were enrolled with moderate-high activity RA (DAS28-CRP >3.2), who have responded inadequately to DMARDs (including methotrexate), after they have been assigned to infliximab treatment. All patients received commercially available infliximab, procured according to SmPC, local guidelines and regulations in this non-interventional clinical study. The clinical response was evaluated according to the change from baseline in disease activity at M6. Clinical characteristics (RA duration, smoke, steroid treatment) and serological parameters (RF, ACPA, aCVM) were collected. A 3rd visit was scheduled around week 22 (M6) and change of DAS28-CRP value from the baseline has been evaluated. Gene expression profiling was performed from blood samples taken at month 0 (M0) – just before the first infliximab infusion. Global gene expression profiling was performed to identify differentially expressing genes using RNA sequencing.
Results: A total of 250 genes were identified by a combination of differential gene expression analyses, feature elimination techniques and various machine learning modelling methods of which 44 genes showed significant differences between NR and good Rs groups The expression of the reduced gene set was confirmed and further analyzed using reverse-transcription and quantitative real-time PCR. Interim analysis identified associations between gene expression and clinical response/ non-response to infliximab therapy.
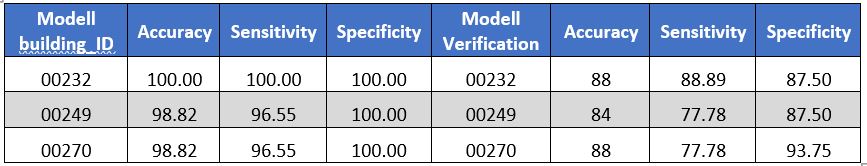
Table: Three models containing gene expression + clinical data sets illustrates some statistical characteristics
Conclusion: This set of genes and selected clinical parameters are predictive markers for infliximab specific response in RA patients. The complete results of the ongoing clinical validation in an independent patient cohort (n=56) is expected in September 2020. This novel in vitro diagnostic test method, for the prediction of infliximab treatment responsiveness before treatment initiation of RA patients, is a tool to personalize infliximab therapy.
To cite this abstract in AMA style:
Sebeszta M, Tauberne Jakab K, Ponyi T, Poor G, Hollo Z, Szilagyi L, Kiss E, Zahuczky G. A Prospective, Multicenter, Clinical Performance Evaluation Study for an in-vitro Diagnostics Medical Device (PREDYSTIC® Infliximab RA) for Prediction of Infliximab Responsiveness in the Treatment of Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/a-prospective-multicenter-clinical-performance-evaluation-study-for-an-in-vitro-diagnostics-medical-device-predystic-infliximab-ra-for-prediction-of-infliximab-responsiveness-in-the-treatmen/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-prospective-multicenter-clinical-performance-evaluation-study-for-an-in-vitro-diagnostics-medical-device-predystic-infliximab-ra-for-prediction-of-infliximab-responsiveness-in-the-treatmen/