Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Because of the altered joint biomechanics seen in nodal hand OA, there may be a role for traction using non-invasive finger traps in this disease. Traction has the potential of providing the benefits of distraction without the attendant complications. We proposed a pilot randomized controlled trial of traction for distal interphalangeal (DIP) nodal hand OA, recruiting patients from the Michael E. DeBakey VA Medical Center and New York University Langone Health.
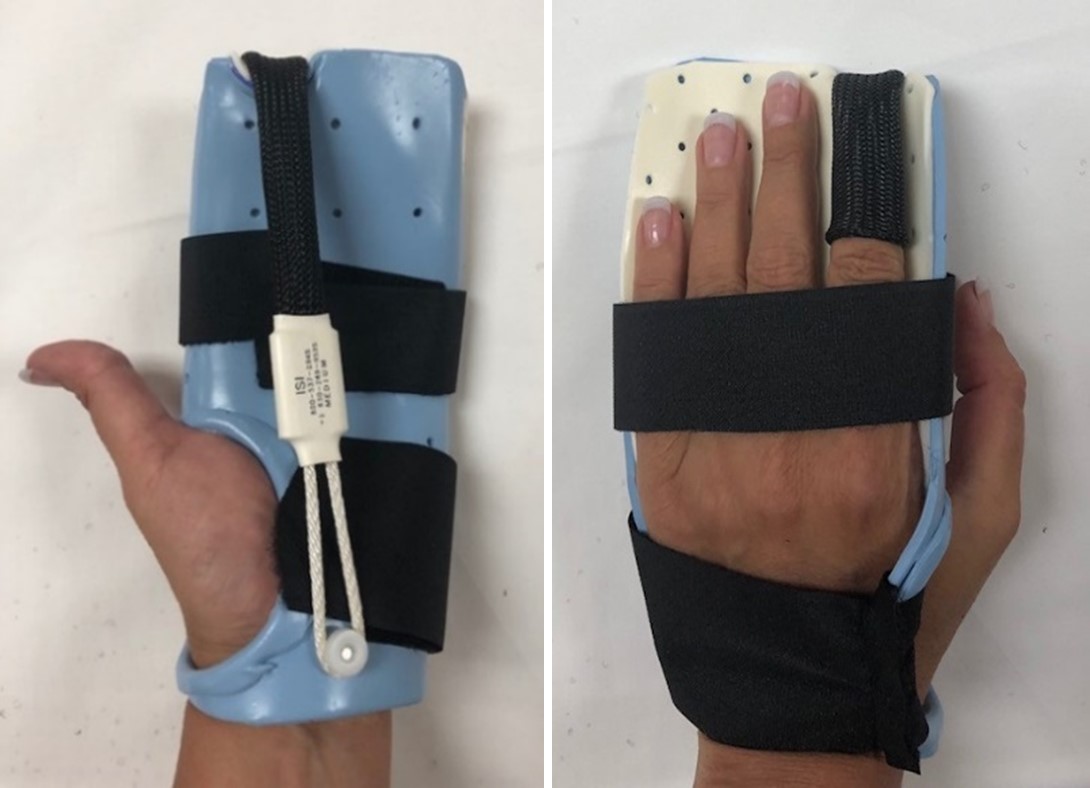
Methods: This was a pilot multi-center, randomized controlled trial of finger traction for established nodal hand OA in at least 3 DIP joints. The control arm received the standard of care for management of hand OA, including a resting orthosis splint, mostly to wear at night. The treatment arm received the same treatment, but also had one or more medical-grade finger traps added to the orthosis to apply overnight finger traction to the most symptomatic DIP joint(s).
Inclusion criteria required evidence of hand OA involving at least 3 DIPs by clinical exam, and sufficiently severe and frequent pain in at least one of those DIP with minimum visual analog score (VAS) of 40 on a 0-100 scale. Exclusion criteria included prior or current inflammatory arthritis, prior or planned surgery of the DIP joints, or current or planned pregnancy.
Participants were invited to come in person visits to conduct screening, baseline, and follow-up visits (weeks 4, 12, and 24). The primary symptom outcome was the 100-point visual analog scale (VAS) in the more symptomatic hand that includes the most symptomatic DIP joint by 24 weeks, comparing the mean change of the participants in traction therapy with standard of care treatment. Secondary outcomes included VAS pain of the most symptomatic DIP, tenderness of the DIP joints, pinch strength, grip strength. Our primary analytic approach was intention- to-treat (ITT) analyses to compare change of VAS pain in the most symptomatic hand between traction therapy and the control groups. Missing outcomes among participants who missed pain and function questions or lost to follow up were addressed by last observation carried forward. Similar analyses were performed for secondary outcomes.
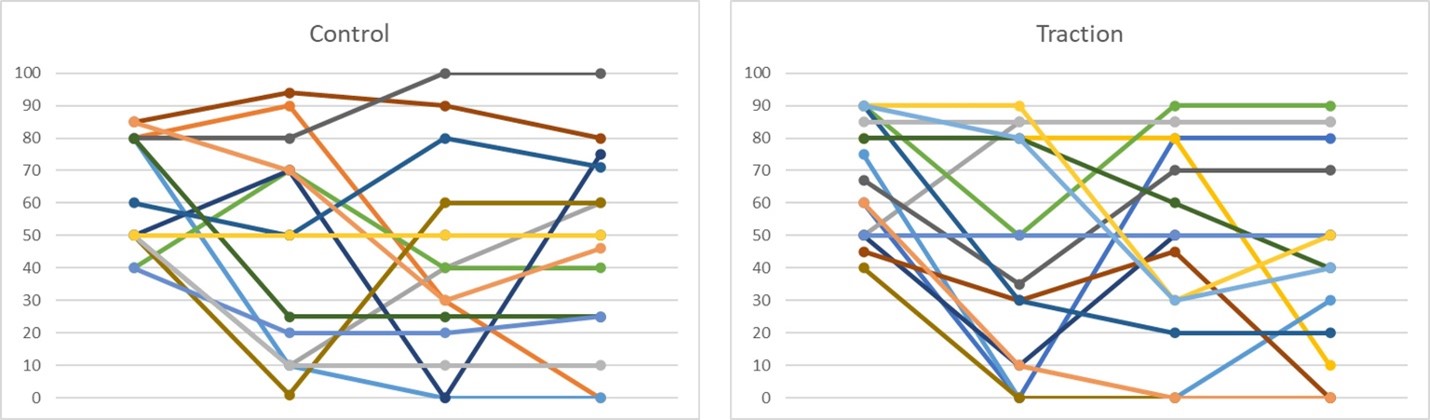
Results: In total 33 participants were randomized into the study, 23 from the MEDVAMC and 10 from NYU. 51% male, 36% Blacks, 17 were randomized to the control arm and 16 to the traction arm. Mean age was 65.9 (6.7) and mean BMI was 32.1 (7.3). Median baseline VAS was 70 for the whole study, 62.5 in the control and 78.0 in the traction arm. The mean change in Study VAS or in the most symptomatic DIP was not statistically different between the two treatment groups.
27 adverse events occurred in 15 subjects in total, 8 in the treatment arm and 7 in the control arm. The most common adverse event was discomfort related to the orthosis. There were no serious adverse events, including death.
Conclusion: Finger traction was a well-tolerated intervention without many adverse events. In this pilot study, we did not find statistically significant difference in our primary outcome of VAS pain in the hand at the 6 months of follow up comparing the traction versus control arm. We plan future additional post hoc analyses to help identify strategies to improve the study design for our larger follow up study.
To cite this abstract in AMA style:
Lo G, Staines K, Strayhorn M, Tse K, Virtanen G, Welsh L, Seu M, Gersh E, Richardson P, Haugen I, Samuels J. A Pilot Randomized Controlled Trial for Hand Osteoarthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-pilot-randomized-controlled-trial-for-hand-osteoarthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-pilot-randomized-controlled-trial-for-hand-osteoarthritis/