Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: CT-P17 is a recombinant humanized monoclonal antibody that was developed as a biosimilar to the reference product, adalimumab. This was a first in human study of CT-P17 (100 mg/mL) designed to evaluate the safety including immunogenicity and pharmacokinetics (PK) compared to European Union (EU)-reference adalimumab (100 mg/mL) in healthy male subjects
Methods: This was a phase 1, randomized, double-blind, two-arm, parallel group, single dose, active comparator study, designed to evaluate the safety and PK of CT-P17 compared to that of EU-reference adalimumab in healthy male subjects. Healthy male subjects aged 18 to 55 years (N=30) were randomized in a 1:1 Ratio to receive 40 mg of either CT-P17 or EU-reference adalimumab by subcutaneous (SC) injection. The primary objective was to evaluate safety in terms of treatment-emergent adverse events (TEAEs). The secondary objective was to evaluate PK parameters and additional safety including immunogenicity.
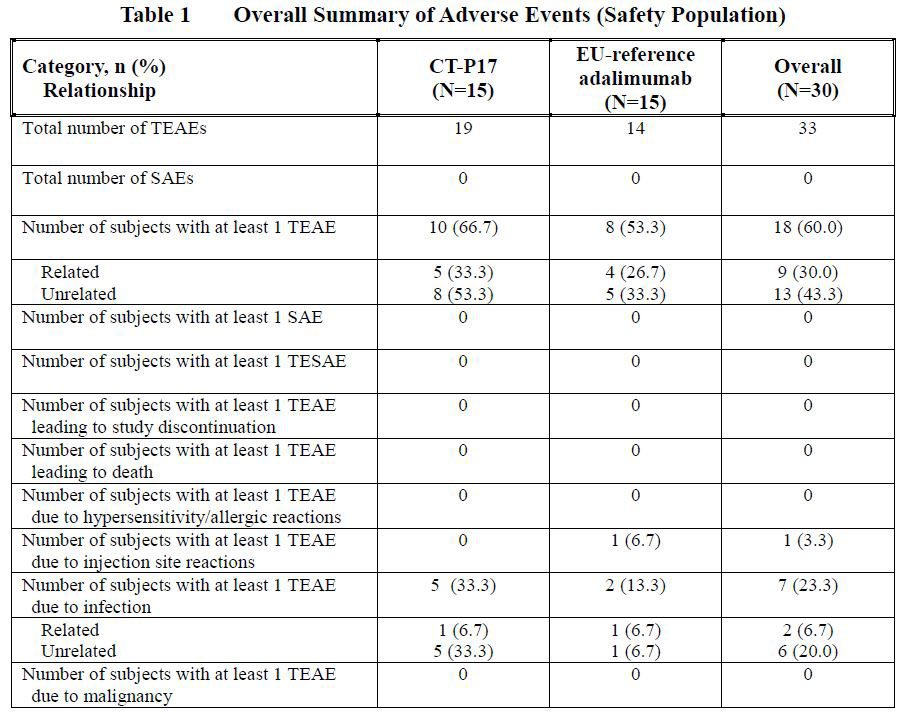
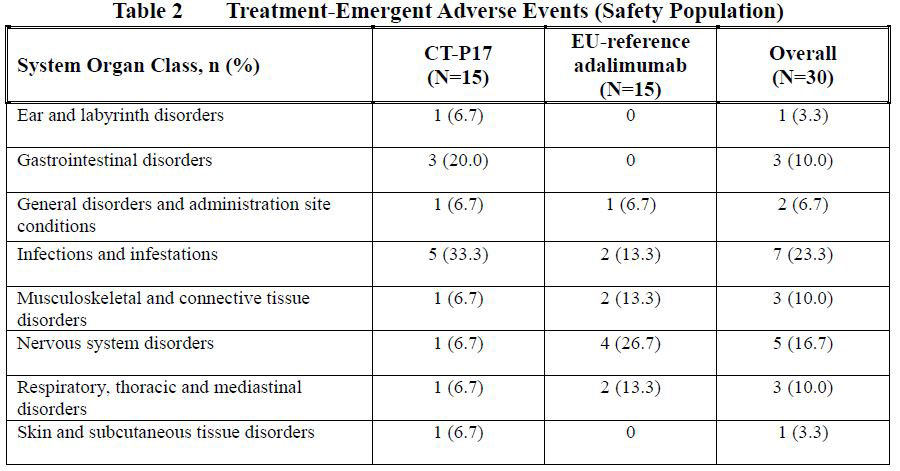
Results: Demographics and baseline characteristics were similar between the 2 treatment groups. Overall, 33 TEAEs were reported and 10 (66.7%) subjects in the CT-P17 and 8 (53.3%) subjects in the EU-reference adalimumab treatment groups reported at least 1 TEAE (Table 1, 2).
The most commonly reported TEAE was nasopharyngitis (4 [26.7%] in CT-P17 and 1 [6.7%] in EU-reference adalimumab). All TEAEs were grade 1 or grade 2 in intensity. There were no deaths, serious AEs, TEAEs leading to study discontinuation, hypersensitivity/allergic reaction or malignancy case for both treatment groups. One (6.7%) subject in the EU-reference adalimumab treatment group reported grade 1 TEAE of injection site reaction. There were no clinically notable abnormalities reported from other safety assessments, including clinical laboratory testing, vital signs, hypersensitivity/allergic reaction monitoring, ECG, physical examination, chest x-ray and tuberculosis assessment.
None of the subjects had a positive anti-drug antibody (ADA) test result at baseline. In the CT-P17 treatment group, 14 (93.3%) and 13 (86.7%) subjects developed at least 1 positive ADA and neutralizing antibodies (NAb) post-dose, respectively. In the EU-reference adalimumab treatment group, 15 (100%) and 14 (93.3%) subjects developed at least 1 positive ADA and NAb post-dose, respectively. Overall, the proportion of subjects with positive ADA and NAb results after study drug administration was similar in the 2 treatment groups.
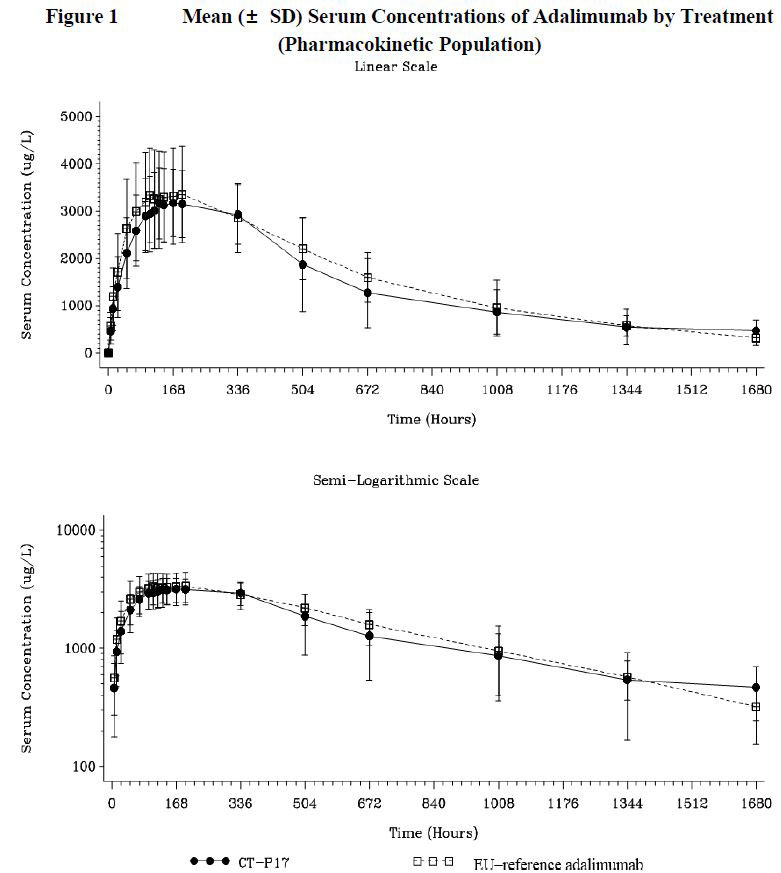
The mean serum concentrations of adalimumab following a single SC dose of 40 mg of CT-P17 or EU-reference adalimumab were comparable up to Day 71 (Figure 1).
The mean values of AUC0-inf were 2383.0 h*ug/mL and 2661.1 h*ug/mL and the mean values of Cmax were 3.415 ug/mL and 3.667 ug/mL for CT-P17 and EU-reference adalimumab, respectively, and were comparable between the 2 treatment groups. All other PK parameters were also comparable between the 2 treatment groups.
Conclusion: Single SC doses of 40 mg of CT-P17 or EU-reference adalimumab were well-tolerated and the safety profile of CT-P17, including immunogenicity, was comparable to that of EU-reference adalimumab in these healthy male subjects.
Pharmacokinetic results were also comparable between the 2 treatment groups.
Note: A subject with 2 or more TEAEs within the same system organ class, preferred term, and relationship is counted only once using the most severe intensity.
To cite this abstract in AMA style:
Keystone E, Furst D, Boyce M, van den Berg F, Lee S, Kim S, Bae Y, Yang G, Koo J, Choi E, Kay J. A Pilot Phase 1, Randomized, Double-blind, Two-arm, Parallel Group, Single-dose Study to Evaluate the Safety and Pharmacokinetics of CT-P17 and Humira in Healthy Male Subjects [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-pilot-phase-1-randomized-double-blind-two-arm-parallel-group-single-dose-study-to-evaluate-the-safety-and-pharmacokinetics-of-ct-p17-and-humira-in-healthy-male-subjects/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-pilot-phase-1-randomized-double-blind-two-arm-parallel-group-single-dose-study-to-evaluate-the-safety-and-pharmacokinetics-of-ct-p17-and-humira-in-healthy-male-subjects/