Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Randomized trials in lupus nephritis (LN) of type I anti-CD20 monoclonal antibodies failed to demonstrate superiority over standard of care alone. NOBILITY (NCT02550652) is a Phase II, randomized, double-blind, placebo (PBO)-controlled study designed to test the hypothesis that enhanced B-cell depletion with the type II anti-CD20 monoclonal antibody obinutuzumab (OBI) will result in improved responses in proliferative LN.
Methods: 125 patients with biopsy-proven ISN/RPS 2003 Class III or IV LN within 6 months and urine protein to creatinine ratio (UPCR) > 1 on a 24-hour collection were randomized to receive OBI 1000 mg or PBO infusions on days 1, 15, 168, and 182 with safety and efficacy assessments through week 104. All patients received mycophenolate mofetil (MMF) and corticosteroids; a corticosteroid taper was mandatory. The primary endpoint was complete renal response (CRR) at week 52, defined as achievement of UPCR < 0.5, normal serum creatinine not increased by > 15% from baseline, and urine RBCs < 10/hpf without RBC casts. Key secondary endpoints were achievement of overall (complete or partial) renal response (ORR), modified CRR without urinary sediment, and improvements in serologic markers of activity. Peripheral B cells were measured using high sensitivity flow cytometry (HSFC). The prespecified alpha level was 0.2.
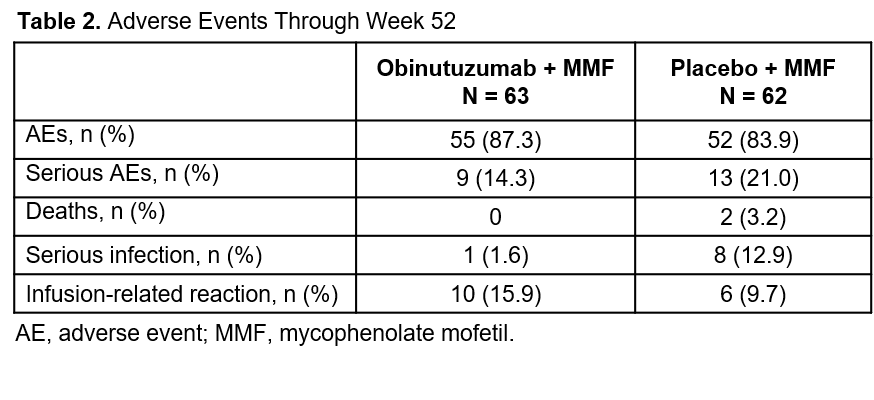
Results: At baseline, mean UPCR was 3.1 g/g and mean serum creatinine was 0.84 mg/dL. The primary endpoint, CRR at week 52, was achieved by 34.9% of patients in the OBI group and 22.6% in the PBO group (12.3% delta; 80% CI 2.1% to 22.6%; P = 0.115). 55.6% of patients in the OBI group and 35.5% in the PBO group achieved ORR at week 52 (20.1% delta; 80% CI 8.9% to 31.3%; P = 0.025). 91% of patients in the OBI group had no detectable peripheral B cells by HSFC at day 28. Significant improvements in anti-dsDNA titers and C3 and C4 levels were observed with OBI compared with PBO. There were no unexpected safety findings. OBI was not associated with increased rates of serious adverse events (14.3% vs. 21.0%) or serious infections (1.6% vs. 12.9%) compared with PBO. Infusion-related reactions were more common with OBI (15.9% vs. 9.7%) and were generally mild. Two deaths occurred prior to week 52, both in the PBO group.
Conclusion: NOBILITY met its primary and key secondary efficacy endpoints. At one year, OBI resulted in increased complete and partial renal responses compared with placebo when added to MMF and corticosteroids for the treatment of proliferative LN. OBI was not associated with increases in rates of serious adverse events or serious infections. Forthcoming data through week 104 will permit further assessment of the longer term safety and efficacy of OBI in proliferative LN.
Acknowledgements: Support for third-party writing assistance, furnished by Health Interactions, Inc, was provided by F. Hoffman-La Roche.
To cite this abstract in AMA style:
Furie R, Aroca G, Alvarez A, Fragoso-Loyo H, Zuta Santillán E, Rovin B, Schindler T, Hassan I, Cascino M, Garg J, Malvar A. A Phase II Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Obinutuzumab or Placebo in Combination with Mycophenolate Mofetil in Patients with Active Class III or IV Lupus Nephritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-phase-ii-randomized-double-blind-placebo-controlled-study-to-evaluate-the-efficacy-and-safety-of-obinutuzumab-or-placebo-in-combination-with-mycophenolate-mofetil-in-patients-with-active-class-iii/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-ii-randomized-double-blind-placebo-controlled-study-to-evaluate-the-efficacy-and-safety-of-obinutuzumab-or-placebo-in-combination-with-mycophenolate-mofetil-in-patients-with-active-class-iii/