Session Information
Date: Monday, October 27, 2025
Title: Abstracts: Rheumatoid Arthritis – Treatment I: Preventative and Novel Treatments (1674–1679)
Session Type: Abstract Session
Session Time: 1:00PM-1:15PM
Background/Purpose: Individuals with elevations of serum anti-cyclic citrullinated peptide (anti-CCP) antibodies are at-risk for rheumatoid arthritis (RA). Identification of interventions to prevent RA in anti-CCP-positive individuals is a current priority. Hydroxychloroquine (HCQ) has been used in such individuals without supporting clinical trial data. We conducted a phase 2, randomized, double-masked, placebo-controlled, parallel group trial to evaluate the efficacy and safety of a 12-month course of HCQ to prevent clinically apparent RA (clinical RA) by 36 months. We previously reported results from interim analyses (ACR Convergence abstract 1604); this current report presents the final clinical trial results.
Methods: Individuals at-risk for RA with anti-CCP3 ≥2 times the upper limit of normal (ULN) were assigned to receive HCQ or placebo for 12 months, with up to 24 months of post-drug follow-up. The primary outcome was the development of clinical RA at 36 months. Clinical RA was defined using the 2010 ACR/EULAR RA Classification Criteria with a score of ≥6, or a joint examination with RA-like synovitis with ≥1 erosion identified via x-ray imaging of the hands, wrists, and feet. Evaluation for clinical RA was triggered by the presence on physical examination of ≥1 joint with RA-like synovitis. Secondary outcomes included safety, development of inflammatory arthritis (IA)(≥1 joint with RA-like synovitis), and participant-reported joint symptoms.
Results: Of 144 randomized participants, 71 were assigned to HCQ and 73 to placebo (Table 1). In the modified intent-to-treat population, clinical RA occurred in 21 of 69 (30.4%) participants in the HCQ group and 24 of 73 (32.9%) in the placebo group. The Kaplan-Meier derived risk of clinical RA at 36 months was 0.336 with HCQ and 0.394 with placebo (difference -0.058; 95% confidence interval -0.336 to 0.220; P=0.52) (Figure). Results for IA were similar. The occurrence and severity of joint symptoms were not observed to differ between groups (Table 2). Adverse event incidence was similar between groups.
Conclusion: In this randomized, placebo-controlled trial involving individuals with anti-CCP3 levels ≥2 times the ULN, a 12-month course of HCQ was not effective in reducing rates of clinical RA at 36 months. No differences were observed between groups in the development of IA or symptoms. These findings demonstrate that this duration of HCQ is not beneficial to prevent clinical RA in similar populations. Moreover, given that there are costs and potential toxicities of HCQ the findings of this trial should inform the care of similar populations. Funded by the National Institute of Allergy and Infectious Diseases; ClinicalTrials.gov NCT02603146.
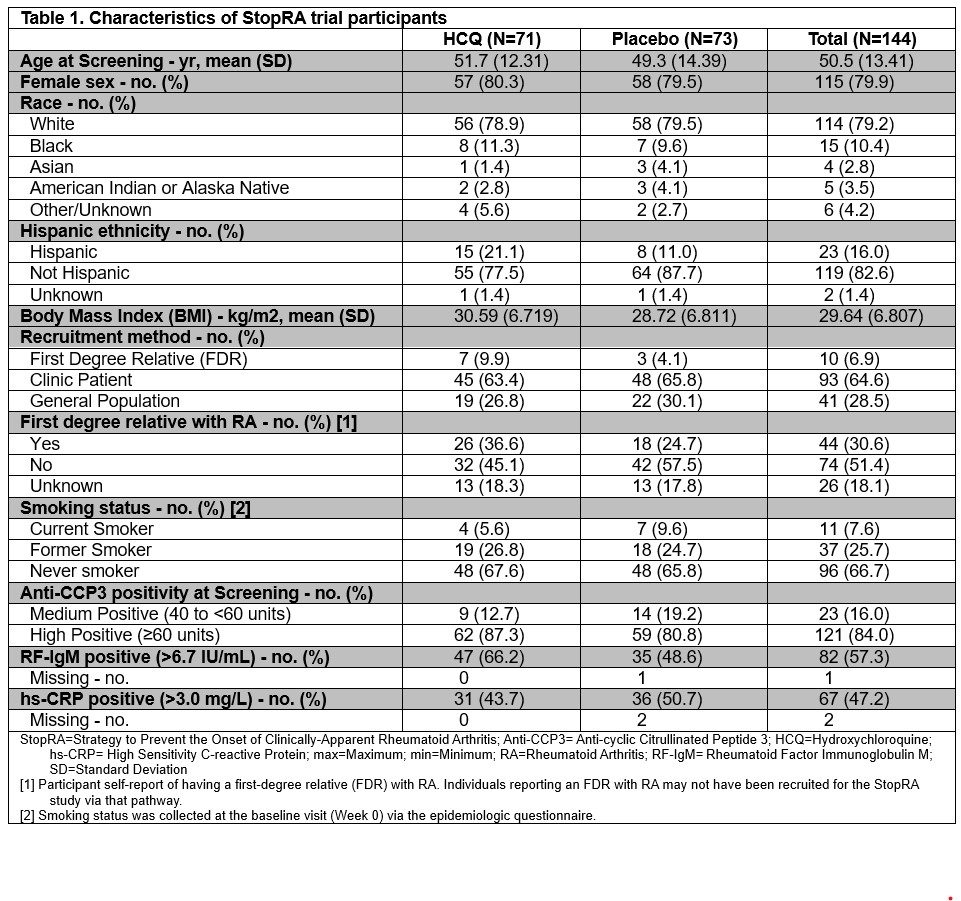 Table 1. Characteristics of StopRA trial participants
Table 1. Characteristics of StopRA trial participants
.jpg) Table 2. Participant-reported joint symptoms over time
Table 2. Participant-reported joint symptoms over time
.jpg) Figure. Development of clinical rheumatoid arthritis. Shown is the Kaplan-Meier based survival estimates of not developing clinical rheumatoid arthritis (clinical RA) in the modified intention-to-treat population. Tick marks indicate censored data. The number of participants at risk for development of clinical RA and the cumulative number of events in each group at each time point is given below the graph. Arrows indicate Month 12 and Month 36. In the modified intent-to-treat population, clinical RA occurred in 21 of 69 (30.4%) participants in the HCQ group and 24 of 73 (32.9%) in the placebo group. The Kaplan-Meier derived risk of clinical RA at 36 months was 0.336 with HCQ and 0.394 with placebo (difference -0.058; 95% confidence interval -0.336 to 0.220; P=0.52).
Figure. Development of clinical rheumatoid arthritis. Shown is the Kaplan-Meier based survival estimates of not developing clinical rheumatoid arthritis (clinical RA) in the modified intention-to-treat population. Tick marks indicate censored data. The number of participants at risk for development of clinical RA and the cumulative number of events in each group at each time point is given below the graph. Arrows indicate Month 12 and Month 36. In the modified intent-to-treat population, clinical RA occurred in 21 of 69 (30.4%) participants in the HCQ group and 24 of 73 (32.9%) in the placebo group. The Kaplan-Meier derived risk of clinical RA at 36 months was 0.336 with HCQ and 0.394 with placebo (difference -0.058; 95% confidence interval -0.336 to 0.220; P=0.52).
To cite this abstract in AMA style:
Deane K, Striebich C, Feser M, O'Dell J, James J, Sparks J, Davis J, Graf J, McMahon M, Solow E, Forbess L, Tiliakos A, Schiopu E, Fox D, Danila M, Horowitz D, Kay J, Strickland C, Guthridge J, Arriens C, Grossman J, Demoruelle K, Bemis E, Frazer-Abel A, Fleischer C, Mikuls T, Greenleaf M, York K, Walker S, Keyes-Elstein L, Byron M, Fedler J, Goldmuntz E, Holers V. A Phase 2, Randomized, Placebo-Controlled Trial of Hydroxychloroquine in Individuals At-Risk for Future Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-phase-2-randomized-placebo-controlled-trial-of-hydroxychloroquine-in-individuals-at-risk-for-future-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-2-randomized-placebo-controlled-trial-of-hydroxychloroquine-in-individuals-at-risk-for-future-rheumatoid-arthritis/
