Session Information
Session Type: Late-Breaking Abstracts
Background/Purpose:
PF-04171327 is a dissociated agonist of the glucocorticoid receptor (DAGR), a selective high-affinity partial agonist of the GR with potent anti-inflammatory activity at exposures that provide less undesirable effects on bone and glucose metabolism compared with prednisone (pred). The objectives of this study were to determine the DAGR doses at which there was sufficient efficacy at Week 8 compared with PBO and pred 10 mg QD; with comparable changes in bone biomarkers and glucose homeostasis compared with pred 5 mg QD.
Methods:
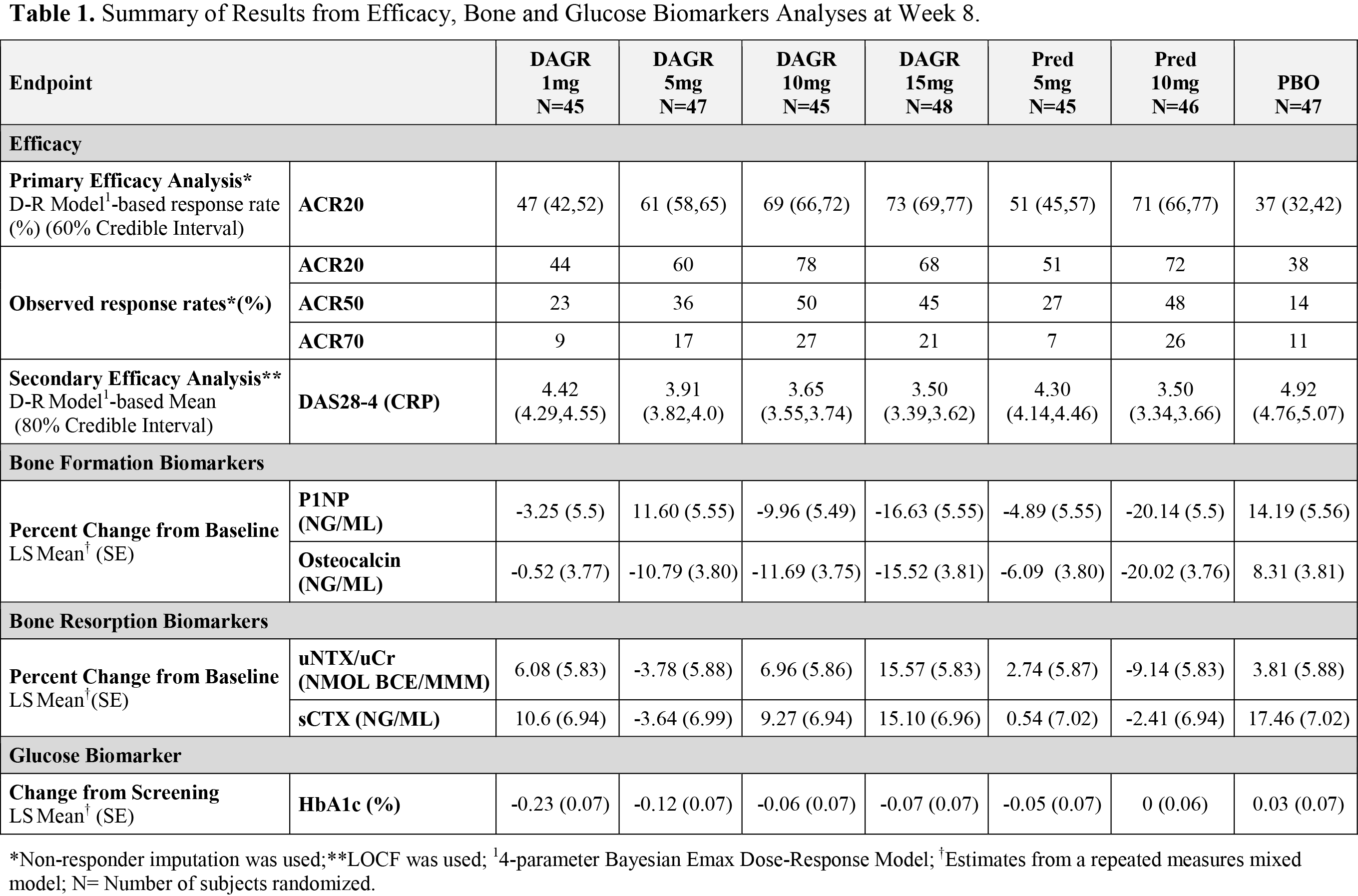
A total of 323 adult patients with active RA (≥6 TJC and 6 SJC plus CRP ≥0.7 mg/dL) receiving MTX were evenly randomized to receive DAGR 1, 5, 10 or 15 mg, pred 5 or 10 mg, or PBO QD for 8 weeks followed by a 4-week taper, and an ACTH stimulation test at Week 13. Use of GCs within 6 weeks of screening was prohibited. Efficacy analyses included a 4-parameter Bayesian Emax model to characterize dose-response (D-R) at Week 8 for primary and secondary efficacy endpoints, ACR20 and DAS28-4(CRP), respectively. In addition to the efficacy endpoints, bone formation (P1NP, osteocalcin) and resorption (uNTX/uCr, sCTX) biomarkers, fasting plasma glucose and HbA1c were collected at protocol specified time points and were analyzed using MMRM methods. AEs and safety laboratory tests were monitored throughout the study.
Results:
Based on ACR20 responses, DAGR 10 and 15 mg QD met criteria for superiority to PBO (by 20% with 80% confidence); and DAGR 15 mg QD met non-inferiority criteria (by a margin of 5% with 80% confidence) to pred 10 mg QD. DAS28-4(CRP) results were consistent with ACR20 results (Table 1). DAGR doses of 1, 5, 10 and 15 mg QD had effects on bone formation markers comparable to pred 5 mg QD; and had small decreases in HbA1c comparable to pred 5mg QD in these non-diabetic RA patients. No clear trends or dose-response were seen in the bone resorption markers. Prompt HPA axis recovery was evident at Week 13 in all patients. All DAGR doses were well-tolerated and comparable to PBO and pred doses, with no safety signal identified.
Conclusion:
The aggregate efficacy analysis demonstrated that in RA patients both DAGR 10 and 15 mg QD have efficacy superior to PBO and comparable to pred 10 mg QD, with effects comparable to pred 5 mg QD on bone formation and glucose markers. All DAGR doses were safe and well-tolerated. Based on the results of this study, further evaluation of
PF-04171327 appears warranted.
Disclosure:
V. Strand,
Idera,
5,
Janssen Pharmaceutica Product, L.P.,
5,
Regeneron,
5,
Lilly,
5,
MerckSerono,
5,
Sanofi-Genzyme,
5,
Novartis Pharmaceutical Corporation,
5,
Pfizer Inc,
5,
Takeda,
5,
Regeneron,
5,
Sanofi-Genzyme,
5,
UCB,
5,
Takeda,
5,
AbbVie,
5,
UCB,
5,
Abbvie,
9,
Amgen,
5,
Abbvie,
9,
Antera,
5,
Amgen,
9,
AstraZeneca,
5,
Anthera,
9,
Biogen Idec,
5,
AstraZeneca,
9,
Anthera,
9,
BMS,
5,
BiogenIdec,
9,
Genetech/Roche,
5,
BMS,
9,
AstraZeneca,
9,
GlaxoSmithKline,
5,
Genentech-Roche,
9,
Idera,
5,
GlaxoSmithKline,
9,
BiogenIdec,
9,
Idera,
9,
Janssen Pharmaceutica Product, L.P.,
5,
Janssen Pharmaceutica Product, L.P.,
9,
Lilly,
5,
Lilly,
9,
BMS,
9,
MerckSerono,
5,
MerckSerono,
9,
Novartis Pharmaceutical Corporation,
5,
Novartis Pharmaceutical Corporation,
9,
Genentech-Roche,
9,
Pfizer Inc,
5,
Pfizer Inc,
9,
Regeneron,
5,
Regeneron,
9,
Sanofi-Genzyme,
5,
GlaxoSmithKline,
9,
Sanofi-Genzyme,
9,
Takeda,
5,
Takeda,
9,
UCB,
5,
Idera,
9,
UCB,
9,
Abbvie,
9,
Amgen,
9,
Janssen Pharmaceutica Product, L.P.,
9,
Anthera,
9,
AstraZeneca,
9,
Lilly,
9,
BiogenIdec,
9,
BMS,
9,
MerckSerono,
9,
Genentech-Roche,
9,
GlaxoSmithKline,
9,
Novartis Pharmaceutical Corporation,
9,
Idera,
9,
Janssen Pharmaceutica Product, L.P.,
9,
Pfizer Inc,
9,
Lilly,
9,
MerckSerono,
9,
Regeneron,
9,
Novartis Pharmaceutical Corporation,
9,
Pfizer Inc,
9,
Sanofi-Genzyme,
9,
Regeneron,
9,
Takeda,
9,
Sanofi-Genzyme,
9,
Takeda,
9,
UCB,
9,
UCB,
9;
F. Buttgereit,
Pfizer Inc,
8,
Pfizer Inc,
6,
Pfizer Inc,
9;
D. McCabe,
Pfizer Inc,
3;
S. Kolluri,
Pfizer Inc,
3;
B. Tammara,
Pfizer Inc,
3;
R. Rojo,
Pfizer Inc,
3;
J. Hey-Hadavi,
Pfizer Inc,
3.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-2-randomized-double-blind-comparison-of-the-efficacy-and-safety-of-pf-04171327-1-5-10-15-mg-qd-vs-5-and-10-mg-prednisone-qd-or-placebo-in-subjects-with-rheumatoid-arthritis-ra-over-8/