Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Globally osteoarthritis affects about 302 million patients, knee osteoarthritis accounts for 263 million and ranks the highest in disability and pain compared to OA of other joints. Efficacious and safe therapeutics for chronic management of OA symptoms are still needed. Topical therapies remain the first line of recommendation for pharmacological management of OA. The current study was to assess a topical gel formulation of XG004, a new chemical entity inhibiting both the COX enzymes and the calcium a2d-subunit, in knee OA patients.
Methods: The Phase 1b study (NCT054540200) was a randomized, double-blind, placebo-controlled, single-center, parallel-group trial, evaluating the safety, PK and efficacy of topical application of XG004 gel in Australian patients with OA of the knee. Patients with moderate to severe OA pain and Kellgren-Lawrence (KL) grade of II-IV were randomized in a 3:1 ratio to receive 4 mL of 5% or 10% topical gel of XG004 or placebo qd, bid, or tid over one week period. Trough plasma and synovial fluid (SF, withdrawn on day 8) PK was assessed with up to 24 hours interval from the last dosing. The exploratory efficacy was measured by changes from baseline in daily walking pain, sleep interference, the WOMAC scores, and the Patient Global Impression of Change (PGIC).
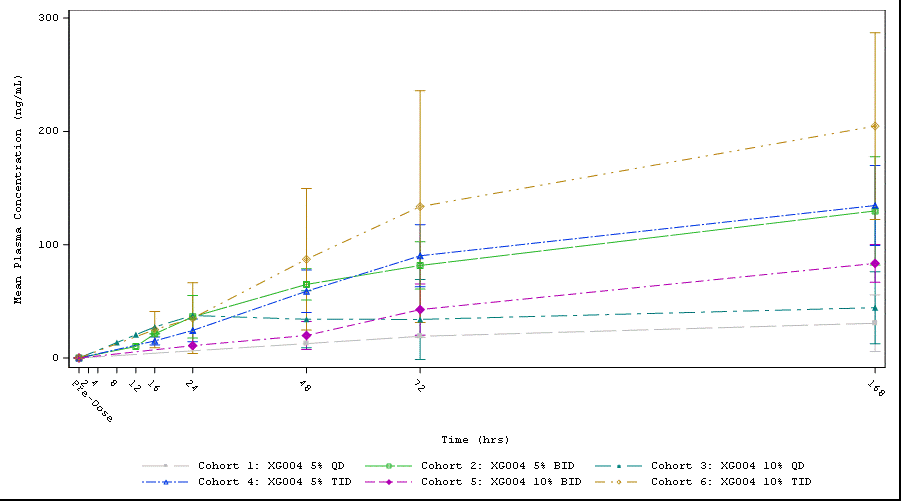
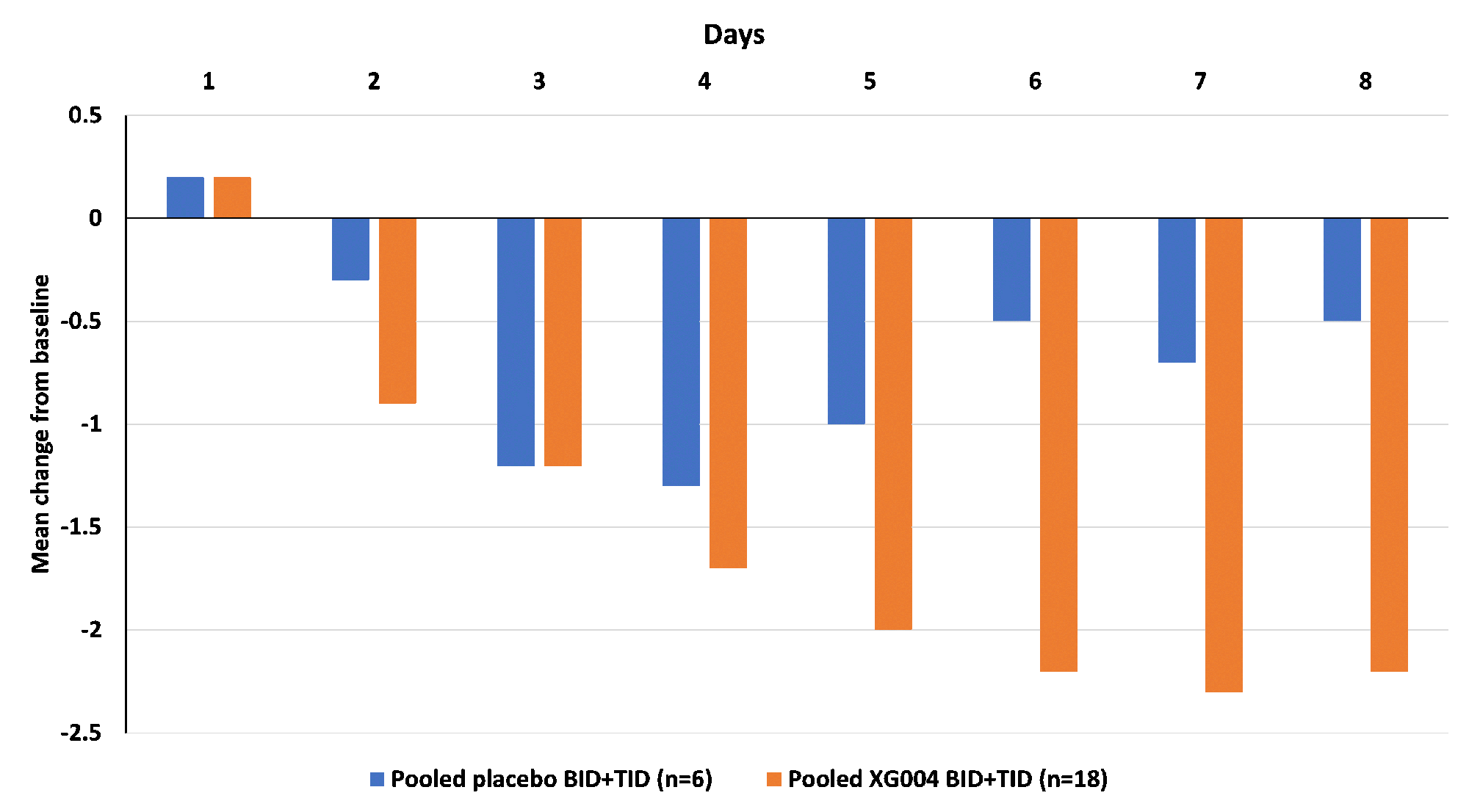
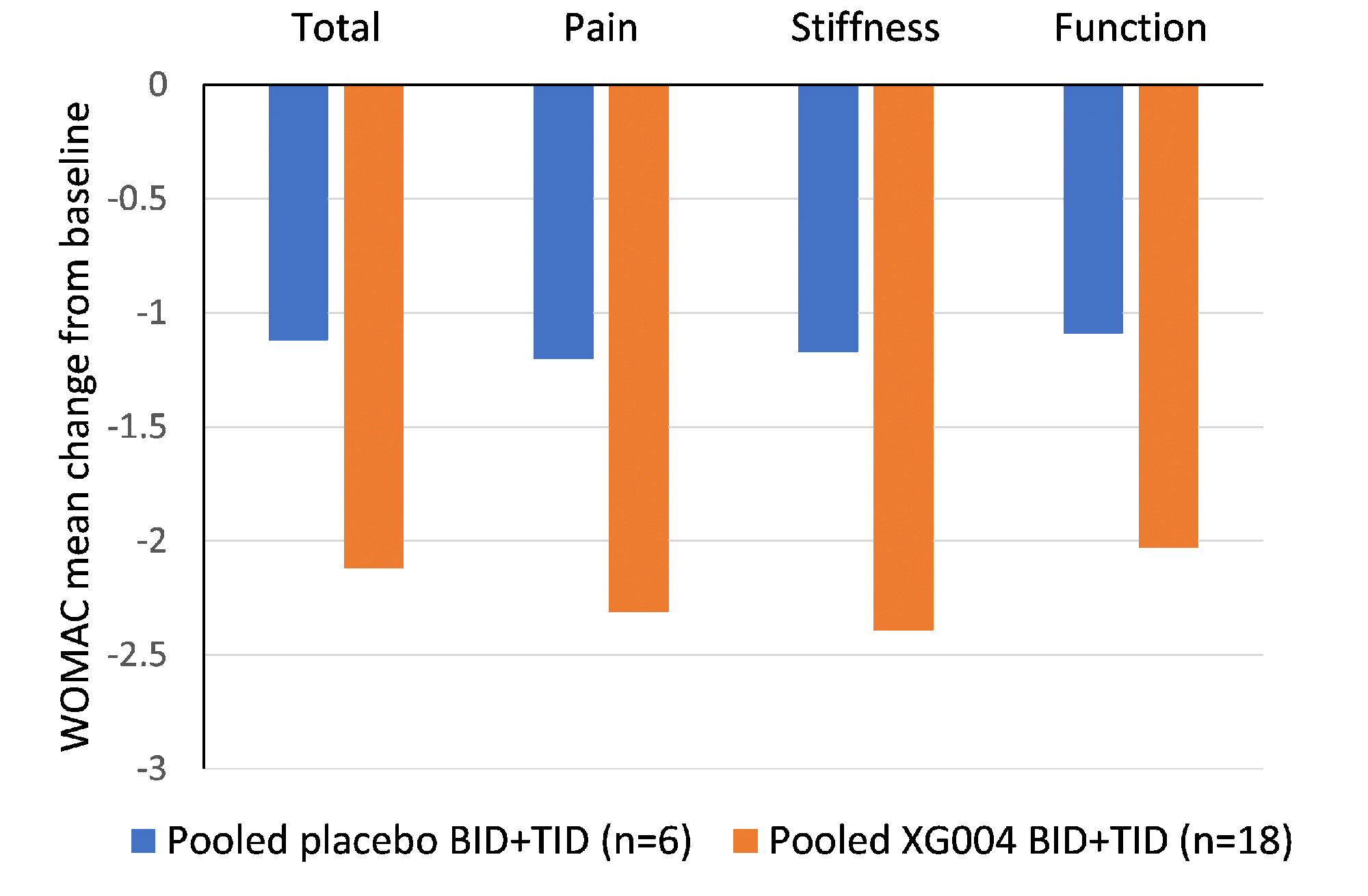
Results: 32 randomized patients had a mean baseline walking pain of 5.7 (0-10 NRS scale), 25% patients had KL grade IV, 56.2% patients had painDETECT score ≥ 13 (indicating neuropathic pain components), those and other baseline characteristics were well balanced between active and placebo arms. All reported good tolerability and safety without SAEs or discontinuation to the study drug. While pregabalin (an active metabolite) was only measurable in plasms of 10% TID dosing cohort, XG004 and another active metabolite naproxen were readily assessable in both plasma and SF. Plasma concentration increased over time in a dose-frequency- and drug-content-dependent way (Fig. 1). The magnitude of symptom improvement was greater in BID or TID dosing cohorts than in the QD cohorts. Over placebo, the BID or TID drug applications resulted in symptom relief in WOMAC pain, physical function, stiffness, total score, and daily walking pain with standard effect sizes of 0.639, 0.444, 0.528, 0.508, and 0.343 by the end of 7 days treatment, respectively (Fig. 3). Meantime, these treatment regimens improved pain-induced sleep interference with an effect size of 0.752 (Fig. 2). 72.2% patients with XG004 BID or TID treatment reported at least “minimally improvement” and 27.8% reported “no change”, while 50% reported at least “minimally improved” and 50% reported “no change” or “minimally worse” in the placebo-treated patients.
Conclusion: XG004 topical gel in knee OA patients showed good tolerability and safety. XG004 and its active metabolites were detectable in sparse plasma and/or SF samples. 5% or 10% BID or TID dosing showed substantial improvement in knee pain, stiffness, physical function, as well as sleep quality. With novel dual targeting on both nociceptive and neuropathic pain signals, XG004 topical could be a promising more efficacious and safer addition to chronic OA pain management.
To cite this abstract in AMA style:
Jiang G, Xu F. A Phase 1b, Randomized, Double-Blind, Placebo-Controlled, Multiple-Dosing, Ascending-Dose Study Evaluating the Safety, Tolerability and Pharmacokinetics of XG004 Applied Topically in Participants with Osteoarthritis of the Knee [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/a-phase-1b-randomized-double-blind-placebo-controlled-multiple-dosing-ascending-dose-study-evaluating-the-safety-tolerability-and-pharmacokinetics-of-xg004-applied-topically-in-participants-with/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-1b-randomized-double-blind-placebo-controlled-multiple-dosing-ascending-dose-study-evaluating-the-safety-tolerability-and-pharmacokinetics-of-xg004-applied-topically-in-participants-with/