Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: A pharmacologic intervention that modulates JAK/STAT signaling pathways represents a novel approach for the treatment of Systemic Lupus Erythematosus (SLE). In animal models of SLE, tofacitinib improved clinical features, immune dysregulation and vascular dysfunction. The STAT4 risk allele is associated with higher risk of severe manifestations in SLE. We hypothesized that immune modulation in response to JAK/STAT inhibition would differ between SLE subjects that carry or not carry the STAT4 risk allele.
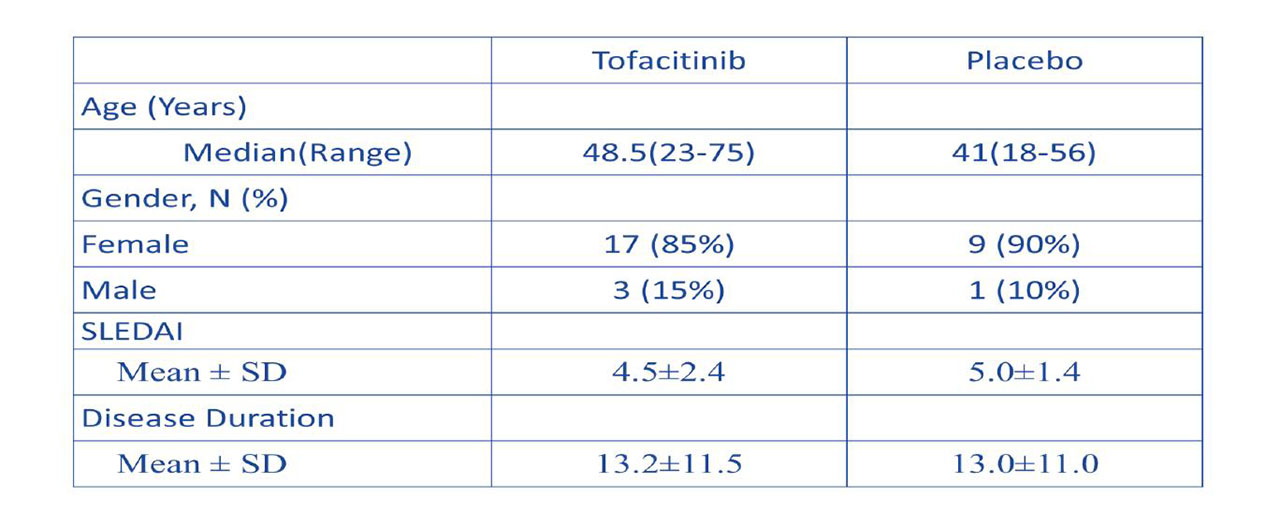
Methods: We conducted a phase 1b/2a randomized, double-blind, placebo-controlled clinical trial of oral tofacitinib, 5 mg twice daily, in 30 SLE subjects (2:1 drug to placebo ratio) with mild to moderate disease activity, stratified by the presence or absence of STAT4 risk allele. Study duration was 84 days, with 56 days of active treatment followed by 28 days of off drug period. In addition to recording adverse events (AEs), lipoprotein profile, non-invasive vascular function studies, immuno-phenotyping, and gene expression studies were performed at various time points.
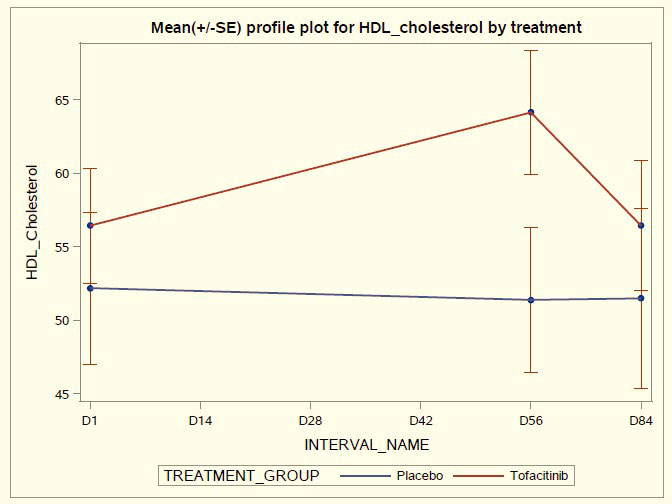
Results: Tofacitinib was well tolerated with no worsening of SLE disease activity, and no severe AEs, opportunistic infections or liver function abnormalities. No thromboembolic events were observed in this short duration trial. A total of 43 AEs (mostly mild respiratory infections) occurred in the treated group compared to 28 AEs in placebo. There was a significant increase in HDL-C and HDL particle size in tofacitinib-treated patients at day 56 accompanied by significant improvements in plasma protein lecithin: cholesterol acyltransferase (LCAT) concentration and cholesterol efflux capacity. Arterial stiffness decreased in the tofacitinib-treated group but not in the placebo-treated group. The type I Interferon gene signature, circulating levels of low- density granulocytes and neutrophil extracellular traps significantly decreased in the tofacitinib treated group compared to the placebo group by the end of treatment, accompanied by significant decreases in pSTAT phosphorylation of different immune cells. Various T cell activation and checkpoint markers significantly decreased in tofacitinib treated individuals and this was modulated by presence or absence of STAT4 risk allele.
Conclusion: In a short-term trial, tofacitinib was well tolerated in SLE subjects with mild-moderate disease activity. Use of tofacitinib resulted in improvements in innate and adaptive immune dysregulation and lipoprotein phenotype and function. This study also points to the potential utility of including genetic data in clinical trials to advance precision medicine. Long-term studies are needed to determine the efficacy of tofacitinib in the various manifestations of SLE including cardiovascular risk.
To cite this abstract in AMA style:
Hasni S, Gupta S, Davis M, Poncio E, Temesgen-Oyelakin Y, Carlucci P, Wang X, Naqi M, Playford M, Goel R, Li X, Biehl A, Ochoa-Navas I, Manna Z, Shi Y, Thomas D, Chen J, Biancotto A, Apps R, Cheung F, Kotliarov Y, Babyak A, Stagliano K, Tsang J, Tsai W, Vian L, Gazaniga N, Giudice V, Brooks S, Mackay M, Gregersen P, Diamond B, Mehta N, Remaley A, O'Shea J, Gadina M, Kaplan M. A Phase 1b/2a Trial of Tofacitinib, an Oral Janus Kinase Inhibitor, in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-phase-1b-2a-trial-of-tofacitinib-an-oral-janus-kinase-inhibitor-in-systemic-lupus-erythematosus/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-1b-2a-trial-of-tofacitinib-an-oral-janus-kinase-inhibitor-in-systemic-lupus-erythematosus/