Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Systemic sclerosis (SSc), a rare autoimmune condition, has a high chronic symptom burden that can have dramatic, life-altering effects on function and quality of life. Fatigue is one of the most problematic symptoms. Pain, depression, and social isolation are also potential contributors to fatigue and reduced quality of life. Our team developed a resilience-based energy management program (called RENEW) designed to be delivered through a website or app. Trained peer health coaches, who also have SSc, provided health coaching during regular online sessions over a 12-week period. This study examined whether participants in the RENEW program had clinically important improvements in fatigue, pain interference, depressive symptoms, and resilience.
Methods: In this randomized controlled trial (NCT04908943), participants were assigned to either RENEW or a wait list control group in a 2:1 ratio. Participants were ≥18 years old with doctor-diagnosed SSc of any subtype. Eligible participants had moderate to severe fatigue (≥4 on the Fatigue Severity Scale). The RENEW program included a comprehensive educational website and app with instructions for goal setting around specific topics plus 9 one-on-one health coaching sessions via Zoom over 12 weeks. The primary outcome was fatigue (assessed by PROMIS FACIT-Fatigue). Secondary outcomes were PROMIS measures of pain interference and depressive symptoms, and Connor-Davidson Resilience Scale. Outcomes were assessed at baseline, 6 weeks, and 12 weeks. Intent-to-treat linear mixed models were used to assess group differences over time, controlling for age, sex, race, SSc subtype, SSc duration, and baseline values of each outcome. A three-way interaction with group, time, and SSc duration was examined in each model.
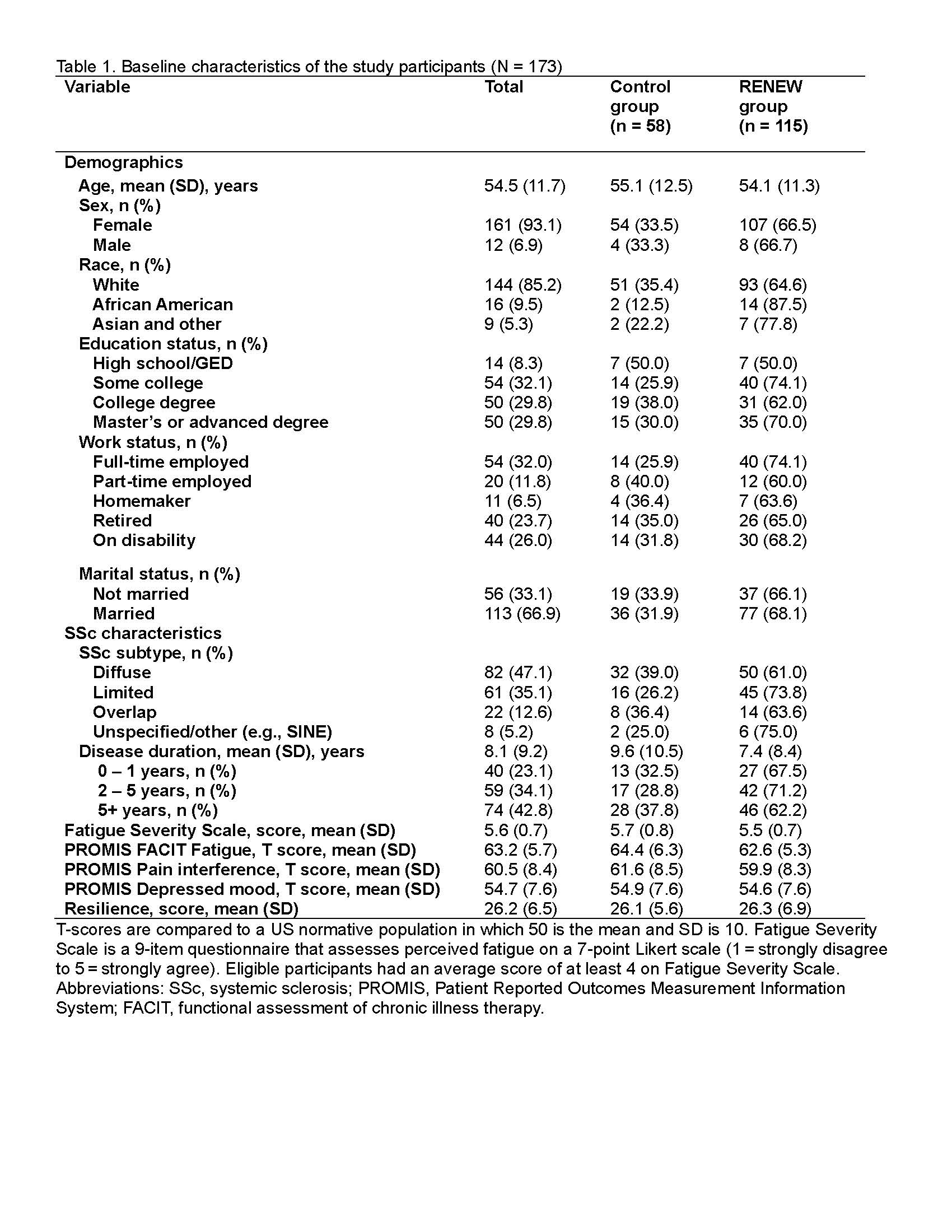
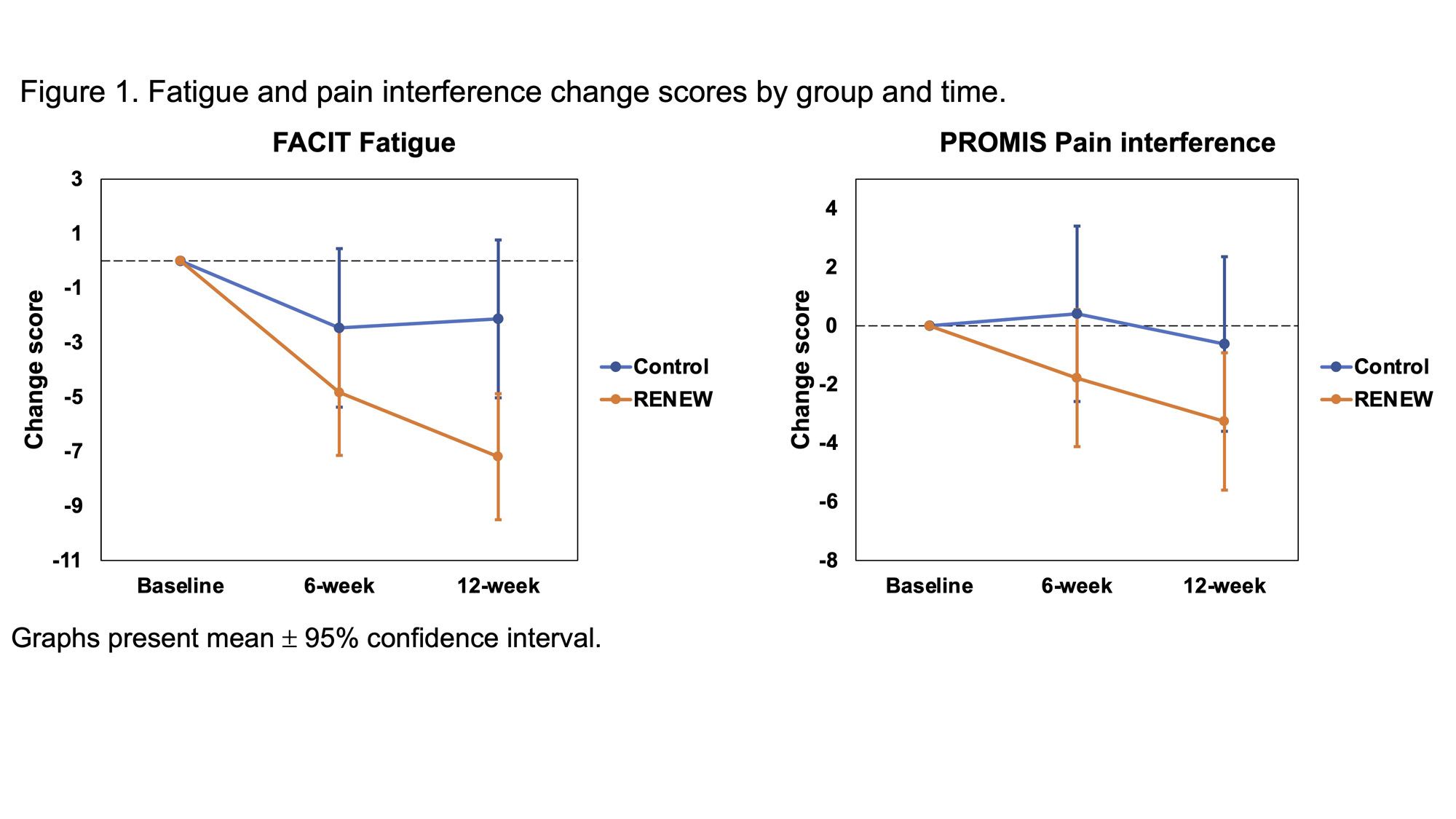
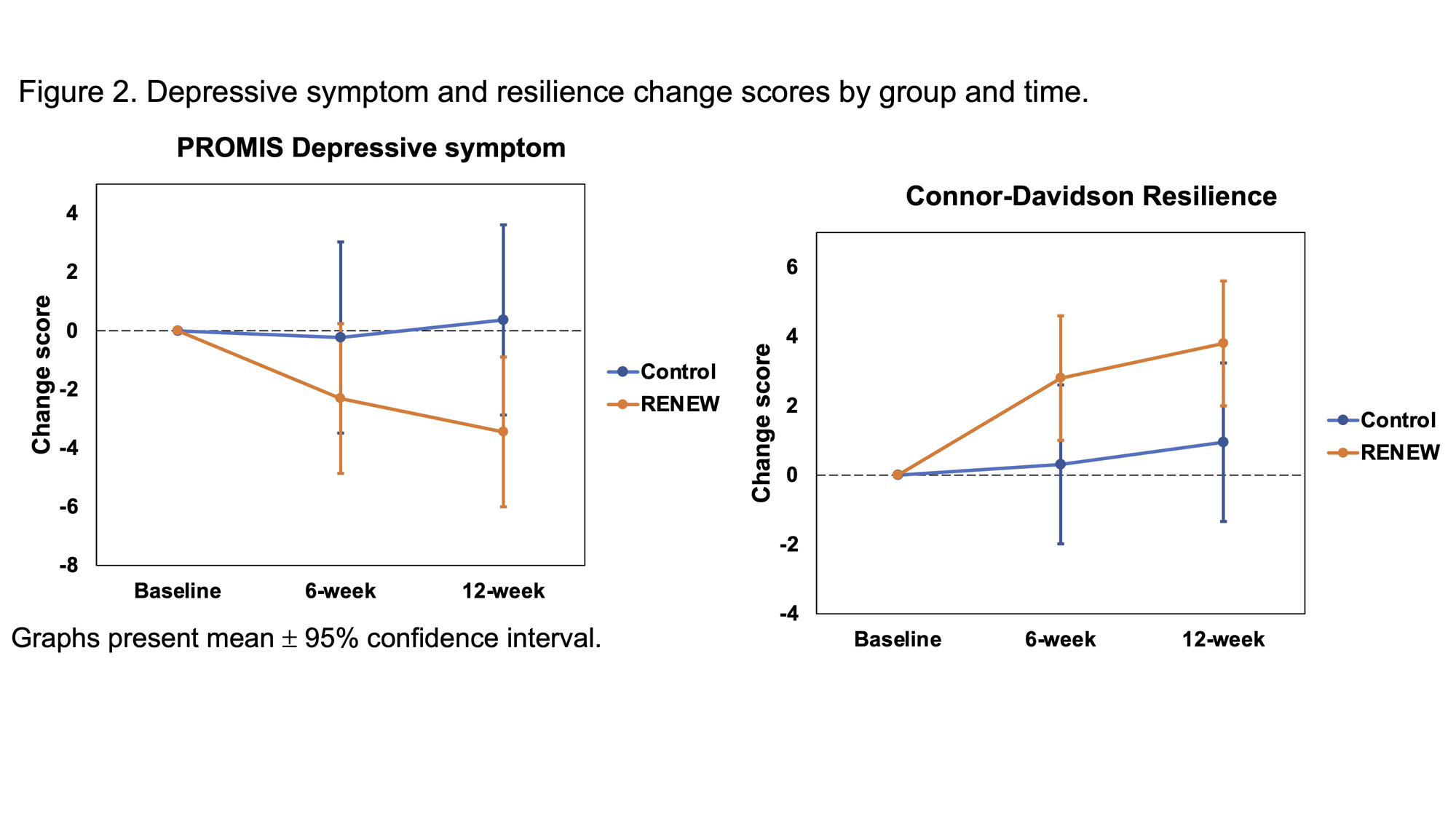
Results: Among 173 participants (mean age 54.5 years ± 11.7), most were female (93%) and White (85%). 47% had diffuse SSc, and 57% had early SSc duration (≤5 years diagnosed) (Table 1). At 12 weeks, participants in RENEW had significantly reduced fatigue compared to controls (β=-5.1, 95% CI: -7.2 to -2.9; p < 0.001), which was considered clinically meaningful ( >3 point change). Participants also had significantly improved pain interference (β=-2.6, 95% CI -4.9 to -0.4; p = 0.021), depressive symptoms (β=-3.8, 95% CI: -6.2 to -1.5; p = 0.002), and resilience (β=2.9, 95% CI: 1.2 to 4.5; p < 0.001) compared to controls (Figures 1 & 2). Within the RENEW group, fatigue and pain at 12 weeks were found to be significantly moderated by SSc duration. Participants with ≤ 1 year SSc duration had significantly reduced fatigue compared to those with >5 year SSc duration (β=-3.1, 95% CI: -5.9 to -0.3; p = 0.032). Participants with 2-5- year SSc duration had significantly reduced pain compared to those with >5 year SSc duration (β=-4.2, 95% CI: -7.0 to -1.4; p = 0.004). No other three-way interaction effects were found.
Conclusion: A remotely-delivered, peer health coached energy management program had positive effects on fatigue and other outcomes, particularly in early diagnosed patients. This program has the potential for broad scalability to assist with SSc symptom management.
To cite this abstract in AMA style:
Murphy S, Chen Y, Alore M, Hicks S, Pape A, Hassett A, Kratz A, Whibley D, Harper A, Huang S, Jay G, Bolde S, Khanna D. A Peer Health Coached Resilience-Based Energy Management Program Was Effective in Improving Fatigue and Other Outcomes in People with Systemic Sclerosis: Results of a Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/a-peer-health-coached-resilience-based-energy-management-program-was-effective-in-improving-fatigue-and-other-outcomes-in-people-with-systemic-sclerosis-results-of-a-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-peer-health-coached-resilience-based-energy-management-program-was-effective-in-improving-fatigue-and-other-outcomes-in-people-with-systemic-sclerosis-results-of-a-randomized-controlled-trial/