Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The success of randomised controlled trials (RCTs) in SLE has been hampered by limitations of current outcome measures, contributing to negative or discordant trial outcomes. To address this, an international Treatment Response Measure for SLE (TRM-SLE) clinician-industry-patient taskforce is developing a new clinical outcome assessment (COA) specifically for use in SLE RCTs (1). The aim of the current study was to achieve consensus on domains of active SLE to include, then identify candidate domain measures that will define clinically meaningful improvement in the new instrument.
Methods: Representatives of key stakeholder groups participated in a modified Delphi study rating potential domains for inclusion, based on domain importance, and utility in an RCT context. Working groups were then established to address the measurement of each domain, comprising adult and paediatric expert clinicians (rheumatologists and relevant specialists), patients with lived experience of domain-specific disease activity, and industry representatives with trial and regulatory expertise. For each domain, working groups sought agreement on domain definition and scope, nominated candidate domain measures via an online process, then shortlisted measures via online consensus meetings, based on their suitability for the measurement goals of the project and RCT context.
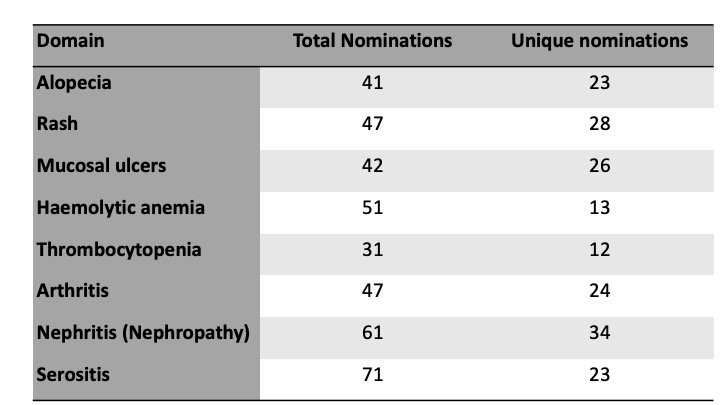
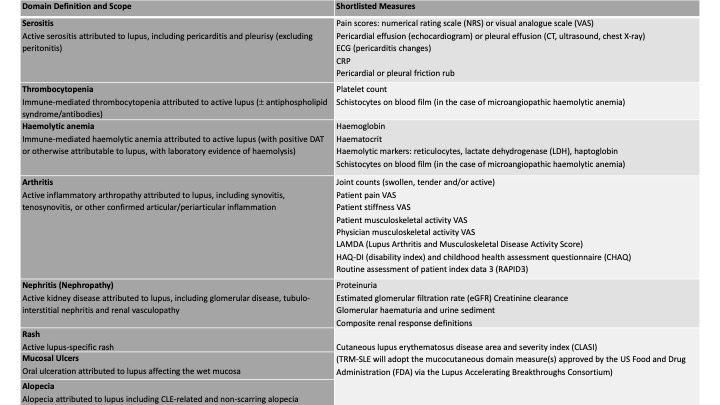
Results: 131 experts (clinicians, patients and industry representatives) from six continents participated. The domains of alopecia, arthritis, haemolytic anemia, thrombocytopenia, mucosal ulcers, nephritis, rash and serositis met the pre-defined consensus threshold for inclusion. The definition and scope of each domain was established, with 100% agreement in each case, focusing on attribution to active SLE, reversibility, and impact on patients’ lives. A total of 391 nominations for candidate domain measures were received across the eight domains, representing 183 unique suggestions (Table 1). Via online consensus meetings, candidate domain measures were shortlisted for each domain, including clinical and laboratory assessments and composite measures (Table 2).
Conclusion: Consensus was achieved across clinician, patient, and industry stakeholders on eight organ domains for inclusion in a new COA for SLE RCTs. To measure these domains, outcome assessments of domain-specific disease activity were shortlisted. Systematic literature reviews of their measurement properties and prior use in RCTs are currently underway. This will be followed by formal nominal group technique processes to reach consensus on each domain measure, assign scoring methods and set thresholds defining clinically meaningful outcomes that will then be integrated into a final COA that will proceed to validation studies.
1. Connelly K et. al. Nat Rev Rheum 2023. doi: 10.1038/s41584-023-00993-7
To cite this abstract in AMA style:
Connelly K, Koelmeyer R, Ayton D, Rajagopala L, Wahklu A, May J, Barallon R, Kandane-Rathnayake R, Eades L, Gregory k, Morand E. A Novel Treatment Response Measure for SLE Clinical Trials (TRM-SLE): Selection of Domains and Candidate Measures [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-novel-treatment-response-measure-for-sle-clinical-trials-trm-sle-selection-of-domains-and-candidate-measures/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-novel-treatment-response-measure-for-sle-clinical-trials-trm-sle-selection-of-domains-and-candidate-measures/