Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Systemic sclerosis-interstitial lung disease (SSc-ILD) is the leading cause of SSc-related death. While some patients respond favorably to treatment with immunosuppression, a subset of patients will experience ILD progression despite treatment. Early loss of lung function, within the first 2 years of treatment, is an independent predictor of mortality. Currently, no evidence-based tools exist to identify patients with a progressive SSc-ILD phenotype. The purpose of this study was to create a prediction tool to identify those patients who are less likely to experience an improvement in ILD in response to treatment with mycophenolate (MMF) or cyclophosphamide (CYC).
Methods: Participants of Scleroderma Lung Study (SLS) II1 were included in this analysis. SLS II randomized patients with SSc-ILD to 2 years of MMF or 1 year of CYC, followed by 1 year of placebo. ILD progression was defined as a >=2% increase in the quantitative extent of ILD in the whole lung (QILD-WL) on HRCT of the chest at 2 years. This radiographic threshold of ILD worsening predicted long-term mortality in both SLS II and SLS I.2 Univariate logistic regression analysis was used to identify baseline variables associated with ILD progression. Baseline variables included demographic characteristics, treatment arm, SSc disease features, ILD severity, reflux severity, and a focused group of serum proteins and auto-antibodies previously found to correlate with extent/progression of SSc-ILD. Those variables associated with outcome (P < 0.1) were then combined into a multivariable model. Receiver-operating characteristic (ROC) analysis was performed. The Bootstrap method was applied to internally validate the model.
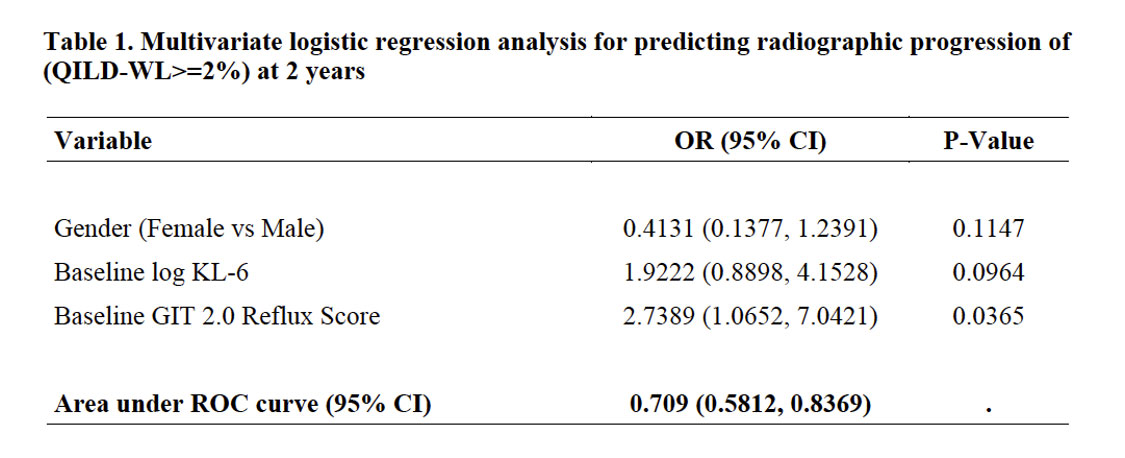
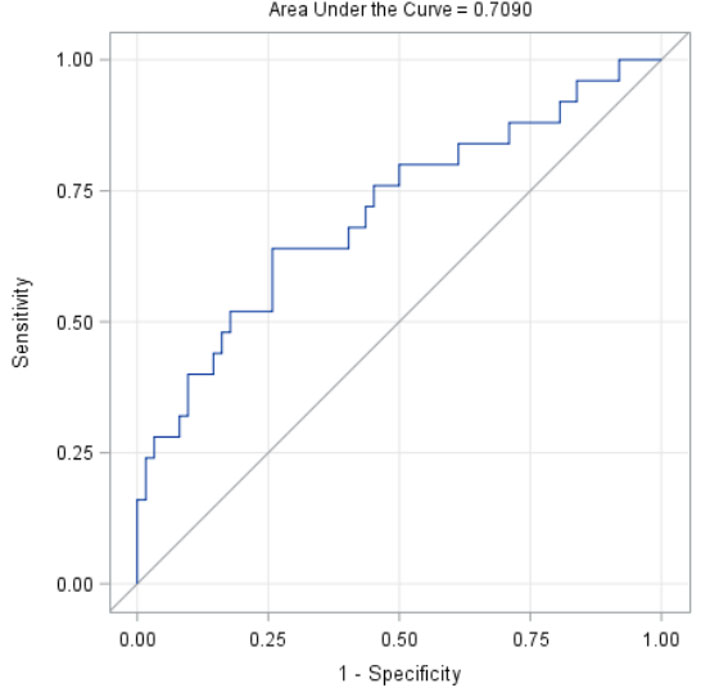
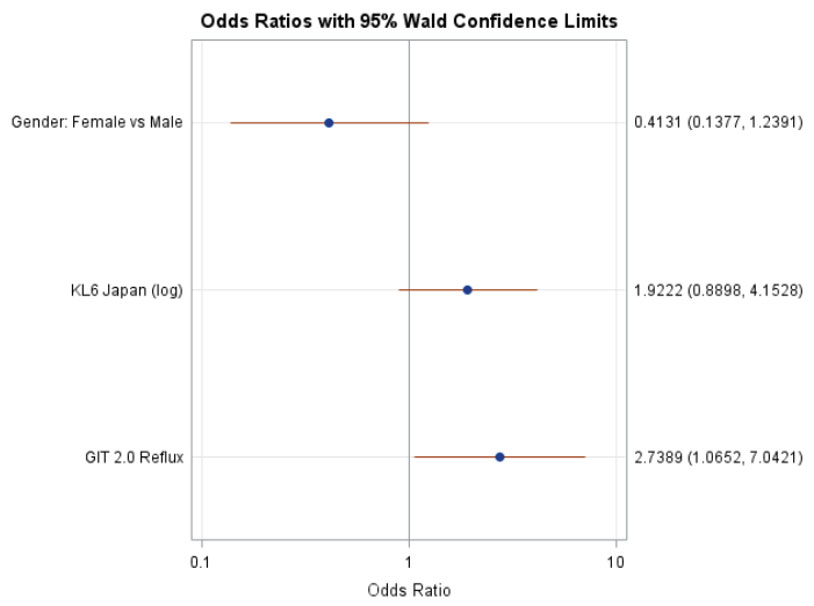
Results: Among SLS II participants who had a follow-up HRCT at 2 years (N=97), 29% experienced radiographic progression of ILD (change in QILD-WL >=2%). There were no significant differences between baseline characteristics of participants with and without the follow up HRCT in SLS II. In the univariate analysis, increased baseline reflux score (P=0.02), increased baseline KL-6 level (P=0.02) and male sex (P=0.05) were associated with worsening of QILD-WL >=2%. The area under the ROC curve for the multivariable model was 0.71 (Table 1; Figure 1). A Forrest plot demonstrates the odds ratios for the individual variables (Figure 2).
Conclusion: A clinical prediction tool defined by male sex, increased KL-6, increased reflux severity had good discrimination for predicting progression of SSc-ILD despite treatment with mycophenolate or cyclophosphamide. Patients with this SSc-ILD phenotype may benefit from a more aggressive initial treatment approach and closer monitoring. This prediction tool requires validation in other cohorts.
References:
1. Tashkin DP, et al. Lancet Resp Med 2016;4:708.
2. Volkmann ER, et al. Chest 2022;161:P1310.
To cite this abstract in AMA style:
Volkmann E, Wilhalme H, Kim G, Goldin J, Assassi S, Kuwana M, Tashkin D, roth M. A Novel Prediction Tool for Progression of Systemic Sclerosis-Interstitial Lung Disease Despite Treatment with Immunosuppression [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/a-novel-prediction-tool-for-progression-of-systemic-sclerosis-interstitial-lung-disease-despite-treatment-with-immunosuppression/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-novel-prediction-tool-for-progression-of-systemic-sclerosis-interstitial-lung-disease-despite-treatment-with-immunosuppression/