Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
C1q is the major subcomponent of the first component of complement protein. The activation of the complement is triggered by binding of C1q to the Fc fragment of immunoglobulin G (IgG). It has been reported that rheumatoid arthritis (RA) patients with high concentrations of C1q in their blood will suffer from joint destruction in the future. Since the activation of C1q is thought to be involved in this joint destruction, binding inhibitor between C1q and IgG will be one of the candidates of new anti-RA drug. The purpose of the present study is to identify the sequence essential for binding between C1q and IgG, to confirm the inhibitory capacity of the peptide with the same sequence, and to verify in a rat collagen-induced arthritis (CIA) model that the peptide has possibility to be a new therapeutic agent for RA.
Methods:
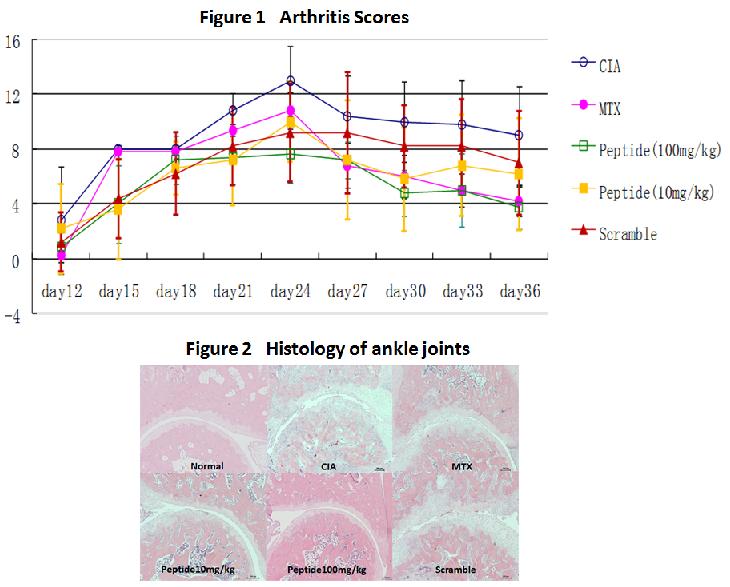
To find the binding inhibitor, we constructed peptide array to identify amino acid sequences of C1q that is crucial for binding between C1q and IgG. Some sequences based on this peptide array analysis were subsequently confirmed to inhibit the binding between C1q and IgG, and the peptide with the most inhibitory sequence (R1 peptide) was determined. For in vivo application, rats with CIA were intraperitoneally injected with R1 peptide or methotrexate (MTX) starting after the onset of arthritis. Disease activity scores, radiographic scores, and histologic scores were evaluated on day 36. Cytokine expressions in the tissue were assessed by realtime RT-PCR. Spleen cell proliferation ex vivo in response to mitogens was examined. Osteoclast formation ex vivo induced by soluble receptor activator of nuclear factor KB ligand (sRANKL) and MCSF was examined. Immunohistochemistry was performed to verify the deposition of IgG and C1q in ankle joints.
Results:
R1 peptide as well as MTX significantly decreased the disease activity scores of CIA. The mean radiographic and histologic scores were significantly lower in the R1-treated rats than in untreated rats. Steady state mRNA levels of TNF-a and IL-1b in ankle joints were decreased in R1-treated rats. There was a significant reduction in phytohemagglutinin-stimulated proliferation of spleen cells in R1-treated rats with the dose of 100 mg/kg day compared to the untreated. Furthermore the osteoclast-like cell differentiation induced by both sRANKL and MCSF was significantly inhibited in R1-treated rats compared to untreated and even MTX-treated rats. Lastly, immunohistochemistry revealed that the deposition of local C1q and was significantly suppressed in R1-treated rats.
Conclusion:
The present study demonstrated that R1 peptide sequence could be essential for binding between C1q and IgG. Furthermore, R1 peptide suppresses the progression of joint destruction in a rat CIA model equivalently to MTX treatment, suggesting the peptide is a potential therapeutic agent for rheumatoid arthritis.
Disclosure:
Y. Moriguichi,
None;
T. Tomita,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-novel-peptide-inhibiting-the-binding-between-c1q-and-immunoglobulin-ameliorates-joint-destruction-in-rats-with-collagen-induced-arthritis/