Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Knee osteoarthritis (KOA) is a frequent disease for which therapeutic possibilities are limited. In current recommendations, the first-line analgesic is acetaminophen. However, its low efficacy frequently leads to the use of weak opioids (WO) despite their poor tolerance, especially in elderly patients.
The primary objective was to compare the analgesic efficacy and safety of a new wearable transcutaneous electrical nerve stimulation (W-TENS) to weak opioids (WO) in the treatment of moderate to severe, nociceptive, chronic pain in KOA patients.
Methods: ArthroTENS study is a non-inferiority, multicentric, prospective, randomized, single-blinded for primary efficacy outcome, controlled, 2-parallel groups, clinical study. It compared W-TENS to WO over a 3-month controlled period with an additional optional non-controlled 3-month follow-up in W-TENS group.
Patients had KOA (ACR criteria) with baseline pain intensity (PI) ≥4 on a numerical rating scale (NRS), after failure to level 1 analgesics/NSAIDs, a Kellgren-Lawrence grade ≥2 and were assessed at baseline, M1 and M3.
The co-primary outcomes were PI at 3-month and the number of treatment-related adverse events (TRAEs) over 3 months. Secondary outcomes included WOMAC function, EuroQol, responder rates defined by PI reduction ≥30 and ≥50% and OMERACT-OARSI response criteria. The non-inferiority margin was defined as 0.825 on PI reduction.
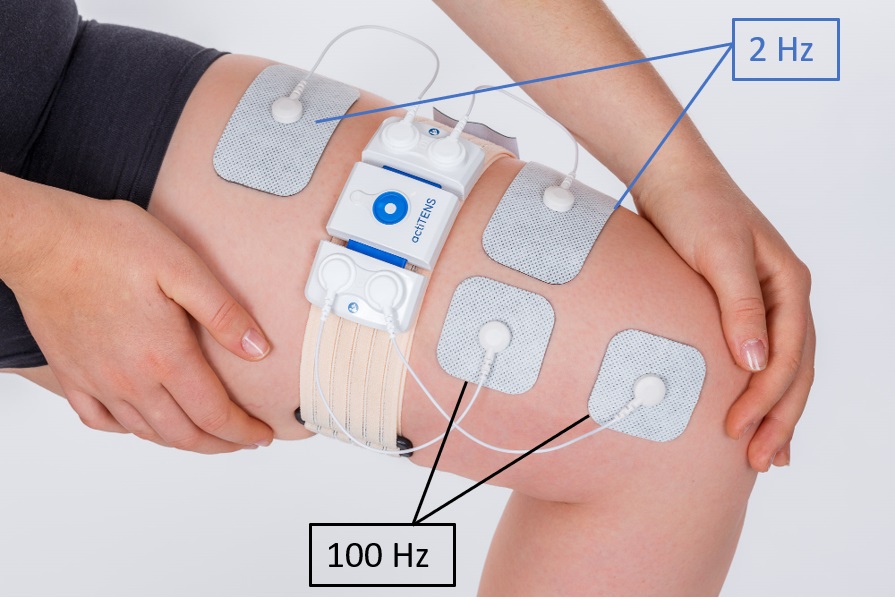
In W-TENS group, an advanced, mobile app enabled, wearable TENS was used. 100Hz and 2 Hz frequency stimulations were delivered via electrodes with standardized positioning (Figure 1).
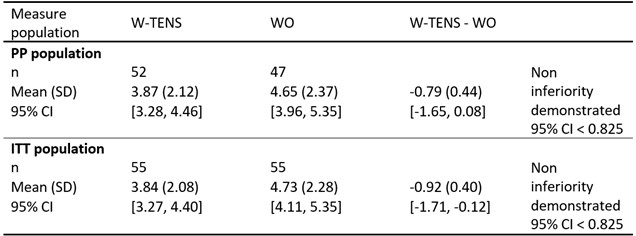
Results: The non-inferiority of W-TENS was demonstrated in both the PP and ITT populations (Table 1). At M3, PI in PP population was 3.87 (2.12) compared to 4.66 (2.37) (delta: -0.79 (0.44); 95% CI (-1.65; 0.08)) in W-TENS and WO groups, respectively. Since the absolute value of the 95% CI of the between-treatments mean PI difference [-1.71, – 0.12] was above 0 in ITT set, the planned superiority analysis was performed, demonstrating that W-TENS was significantly superior to WO at M3 (P=0.0124).
In the ITT population, the number of potentially TRAEs was significantly lower (p< 0.001) in the W-TENS group (n=7) than in the WO group (n=36) during the 3-month controlled follow-up. The AEs observed in the W-TENS group were mainly local cutaneous reactions (erythema: 5.5%) due to the TENS technique, while those observed in the WO group were systemic and well known (dry mouth: 1.8%; vomiting: 1.8%; pruritus: 3.6%; dizziness: 5.5%; drowsiness: 7.3%; nausea: 10.9% and constipation: 12.7%) and limit their use and effectiveness in clinical practice. Thirty-nine (70.9%) patients wished to extend W-TENS treatment for 3 additional months. The efficacy was maintained throughout this additional therapeutic period. All secondary outcomes favored the W-TENS group at M1 and M3.
Conclusion: W-TENS was more effective and better tolerated than WO in the treatment of nociceptive KOA chronic pain and could represent an interesting non-pharmacological analgesic alternative for patients.
To cite this abstract in AMA style:
Maheu E, Soriot-Thomas S, Noël E, Ganry H, Lespessailles E, Cortet B. A New Wearable Transcutaneous Electrical Nerve Stimulation Device (actiTENS®) Is More Efficient and Better Tolerated Than Weak Opioids in the Treatment of Knee Osteoarthritis Pain [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/a-new-wearable-transcutaneous-electrical-nerve-stimulation-device-actitens-is-more-efficient-and-better-tolerated-than-weak-opioids-in-the-treatment-of-knee-osteoarthritis-pain/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-new-wearable-transcutaneous-electrical-nerve-stimulation-device-actitens-is-more-efficient-and-better-tolerated-than-weak-opioids-in-the-treatment-of-knee-osteoarthritis-pain/