Session Information
Date: Monday, November 11, 2019
Title: Metabolic & Crystal Arthropathies Poster II: Clinical Trials & Basic Science
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Hyperuricemia, a common metabolic disorder, predisposes patients to develop gout due to the deposition of insoluble urate in joints. Safety concerns are frequently raised with commonly prescribed uricosurics. The purpose of present trial is to assess the efficacy and safety of SHR4640 – a highly selective URAT1 inhibitor in hyperuricemic subjects in China.
Methods: Subjects with gout and serum uric acid (sUA) ≥480 μmol/L, or subjects without gout but with (sUA ≥480 μmol/L) or without (sUA ≥540 μmol/L) cardiovascular dysfunction were enrolled. All eligible subjects were randomly assigned (1:1:1:1:1) to monotherapy of SHR4640 2.5, 5, 10 mg, benzbromarone 50 mg and placebo, respectively. The primary endpoint was the proportion of subjects achieving sUA target of ≤360 μmol/L after 5-week treatment. This trial was registered with ClinicalTrials.gov, NCT03185793.
Results: A total of 197 subjects received assigned treatments. Subjects were predominantly male (99.5%) and associated with gout (95.9%), with a median age of 41.0 years (range, 18.0 to 69.0). The sUA targets were achieved in 32.5%, 72.5% and 61.5% of subjects receiving SHR4640 5 mg, 10 mg and benzbromarone 50 mg, respectively, which were significantly higher than that of placebo (0%, P < 0.05, Figure 1). The sUA was 596.2 ± 87.5 μmol/L at the baseline, and was reduced by 18.0%, 32.2%, 47.0% and 41.4% after 5-week treatment with SHR4640 2.5 mg, 5 mg, 10 mg and benzbromarone 50 mg, respectively, which were significantly greater than that of placebo (5.8%, P < 0.05, Figure 2). The incidence of gout flare requiring intervention was similar across all groups. SHR4640 was well-tolerated. Occurrences of AEs were comparable across all treatment groups, although the incidence of transient elevated serum creatinine was slightly higher in SHR4640 10 mg as comparing with others (serum creatinine elevations between 1.5-fold and 2-fold over baseline occurred in 5.0% [n=2] of patients on SHR4640 10 mg, 0% in other groups, Table 1). Serious AE including death was not reported.
Conclusion: The present trial demonstrated greater sUA-lowering effect of SHR4640 5 mg and 10 mg versus placebo, with a generally tolerable safety profile. SHR4640 represents a new treatment option for subjects with hyperuricemia in China.
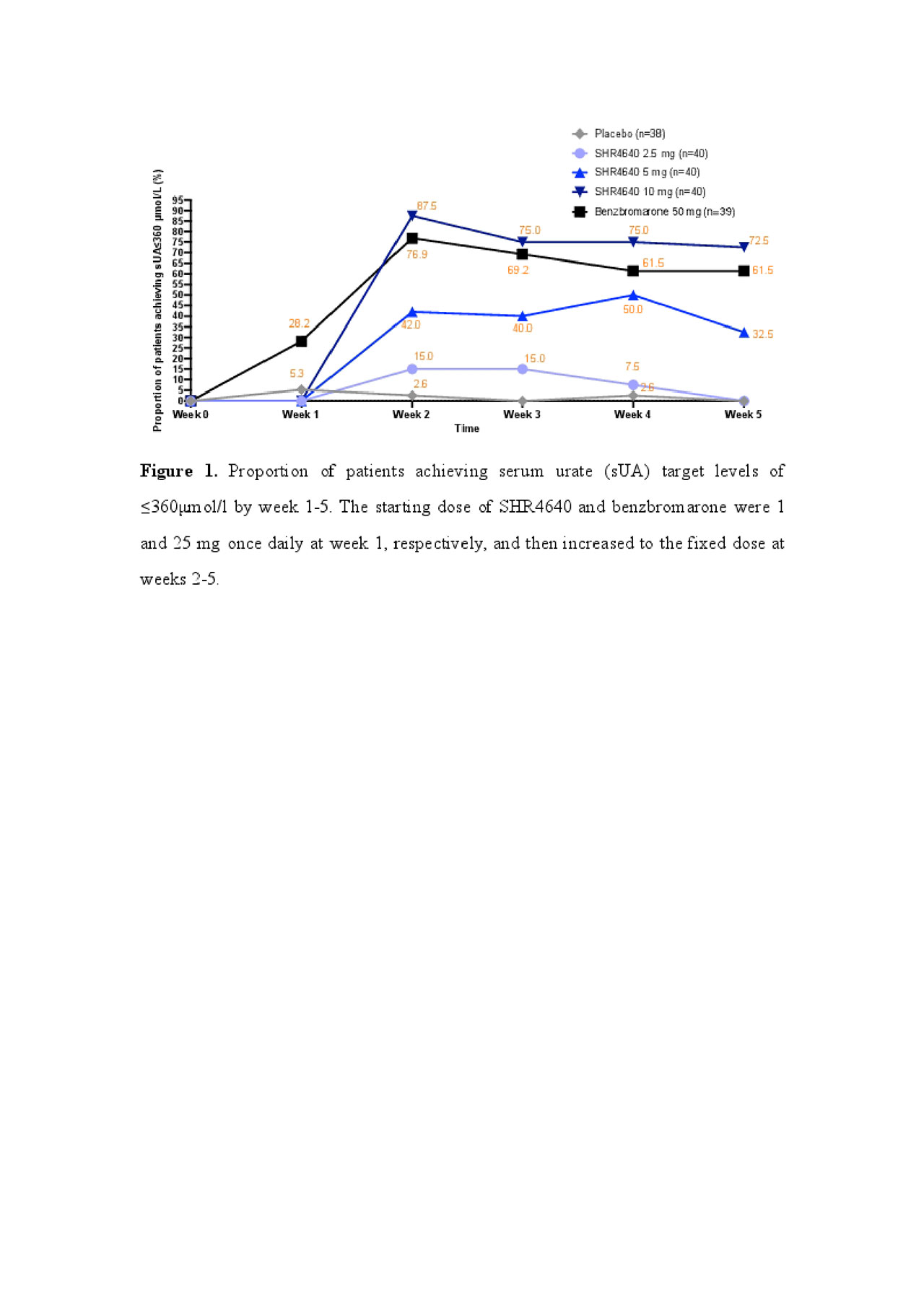
SHR4640-201 ACR abstract Figure 1
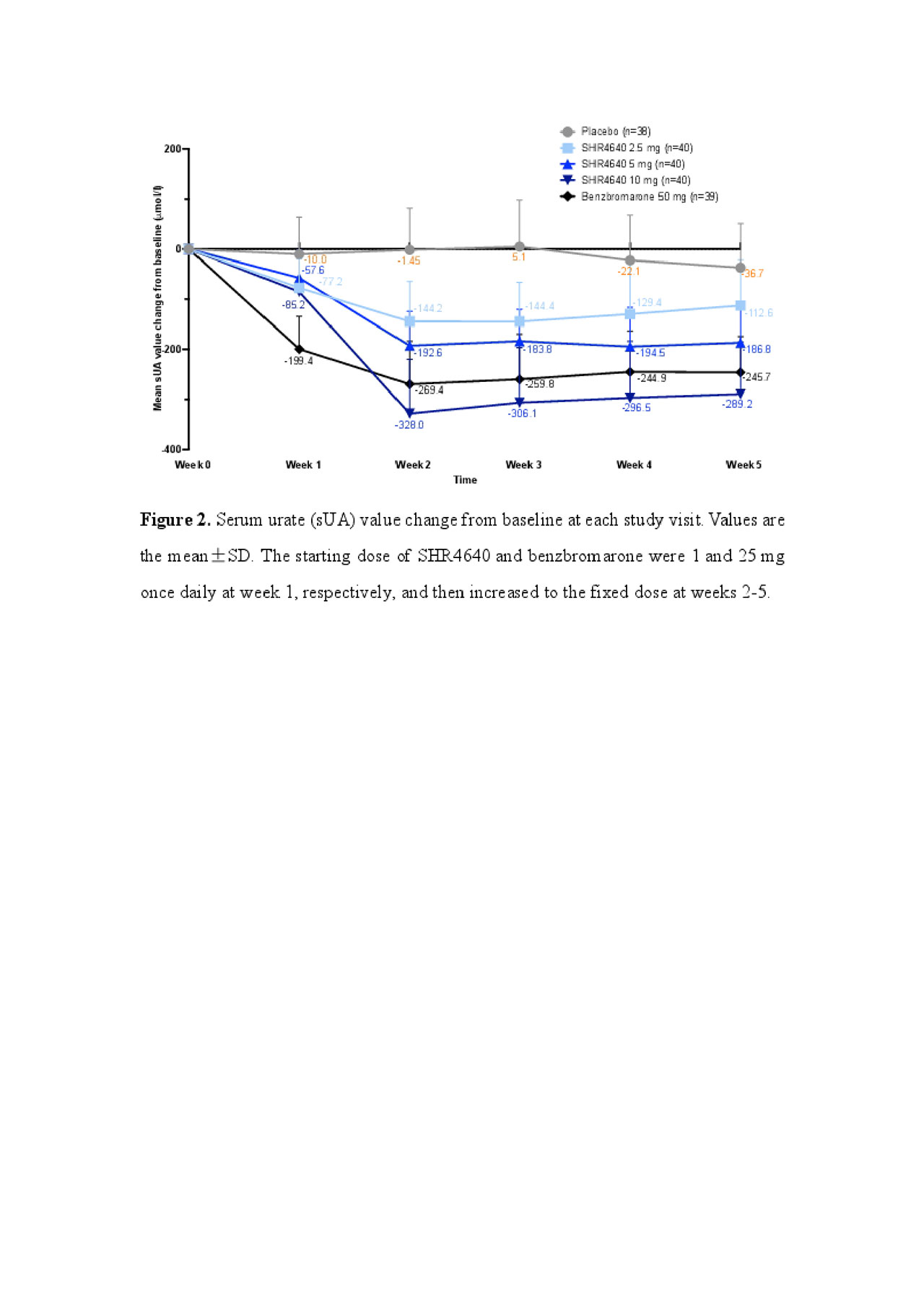
SHR4640-201 ACR abstract Figure 2
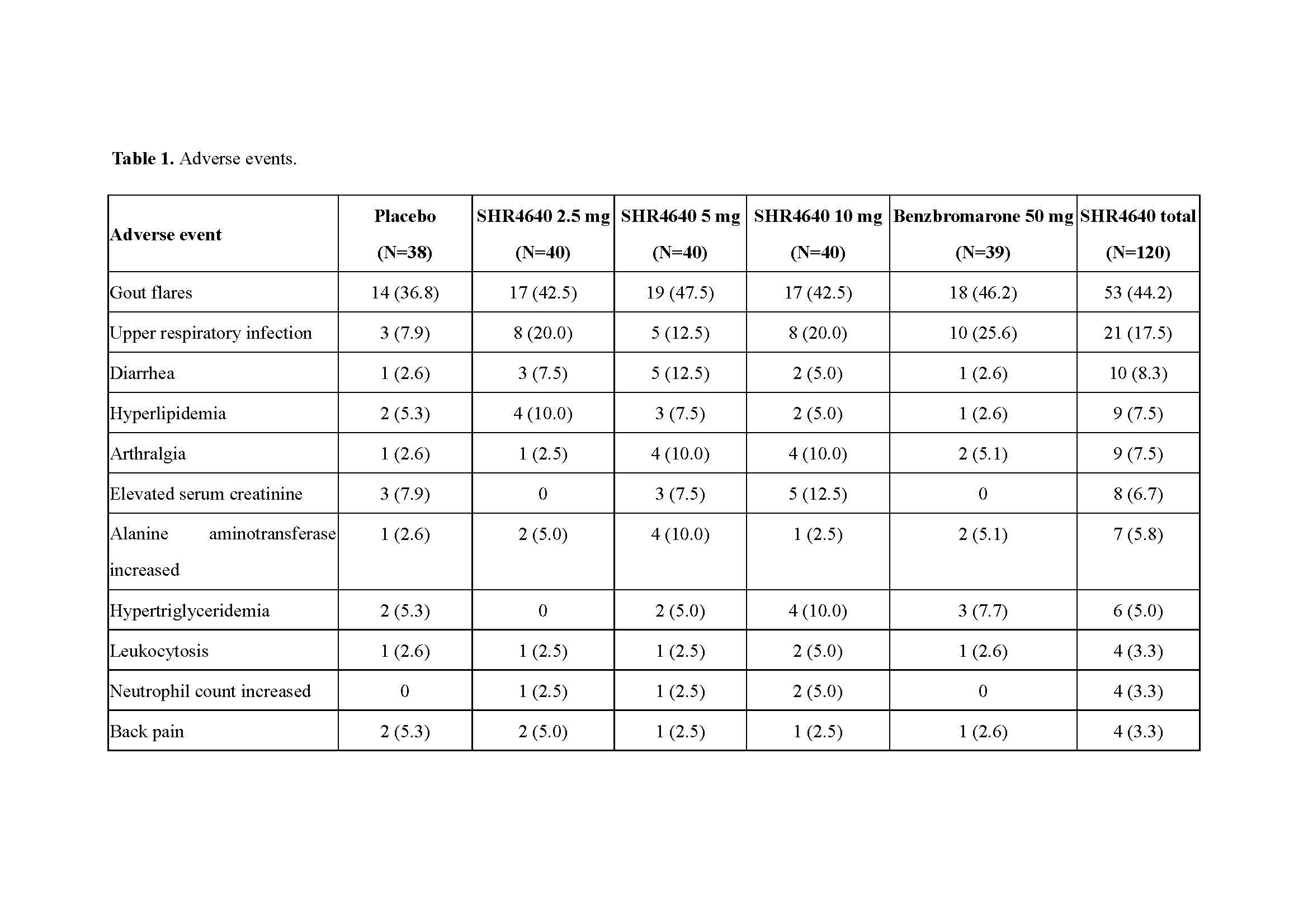
SHR4640-201 ACR abstract Table 1
To cite this abstract in AMA style:
Lin Y, Chen X, Ye P, Geng W, Ning R, Tai Y, Bao C. A Multicenter, Randomized, Double-blind, Placebo-controlled, Dose-Ranging Study to Evaluate Efficacy and Tolerability of SHR4640 in Patients with Hyperuricemia [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-multicenter-randomized-double-blind-placebo-controlled-dose-ranging-study-to-evaluate-efficacy-and-tolerability-of-shr4640-in-patients-with-hyperuricemia/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-multicenter-randomized-double-blind-placebo-controlled-dose-ranging-study-to-evaluate-efficacy-and-tolerability-of-shr4640-in-patients-with-hyperuricemia/
