Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Lorecivivint (LOR), a novel intra-articular (IA) CLK/DYRK inhibitor that modulates Wnt and inflammatory pathways, is in development as a knee osteoarthritis (OA) treatment. To further evaluate the safety and exploratory efficacy of a single LOR injection that was administered into the target knee joint of subjects with moderate to severe knee OA from two consecutive Phase 2 trials, an extension study was performed with the primary objective of evaluating serious adverse events (SAEs), as well as analyzing safety data for all doses and efficacy data for the (pivotal) 0.07 mg LOR dose.
Methods: This was a 5-year, Phase 3, multicenter, observational, extension study of completer subjects (NCT02951026) from a 12-month Phase 2a (Yazici, Y. et al. Arthritis Rheumatol. 72, 1694–1706 (2020)) and a 6-month Phase 2b (Yazici, Y. et al. Osteoarthr. Cartil. In Press, (2021)) trial of LOR. The study was terminated in Year 3 as relevant long-term safety information became limited in the absence of repeated LOR administration. Subjects received a single LOR or vehicle placebo (PBO) injection at their Phase 2 parent-trial baseline visit (Month 0). Pooled data from clinic visits at 6, 12, 24, and 36 months were used to analyze safety outcomes (serious AEs, knee-related AEs, and AEs of newly diagnosed conditions needing treatment). A post hoc baseline-adjusted ANCOVA on 0, 3, 6, 12, and 18-month data points (across current and parent trials) was used to compare changes from baseline in a subject subgroup (unilateral symptoms, no widespread pain, 18-month post-injection radiograph at study termination) in WOMAC Pain and Function subscores and medial joint space width (mJSW) between 0.07 mg LOR and PBO groups.
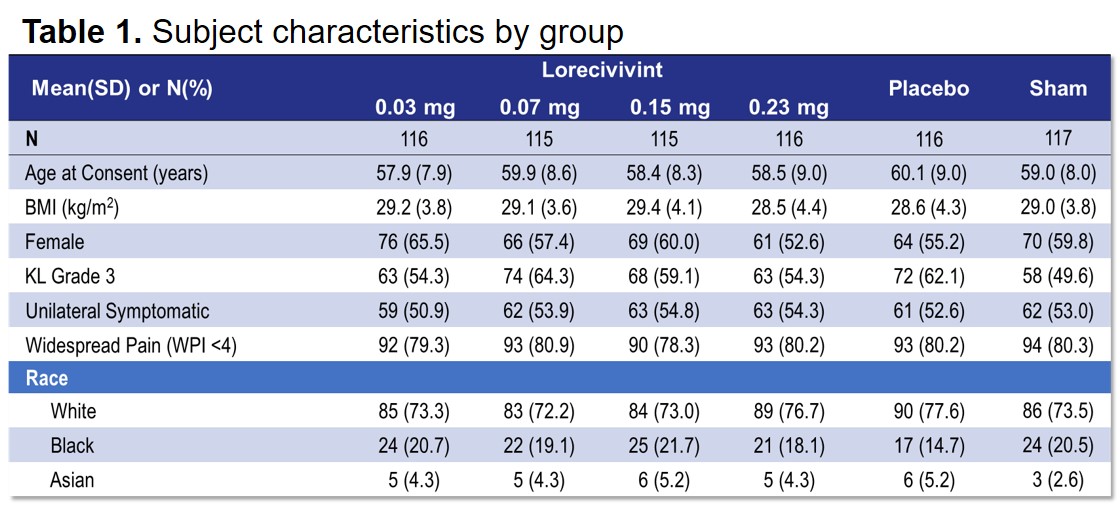
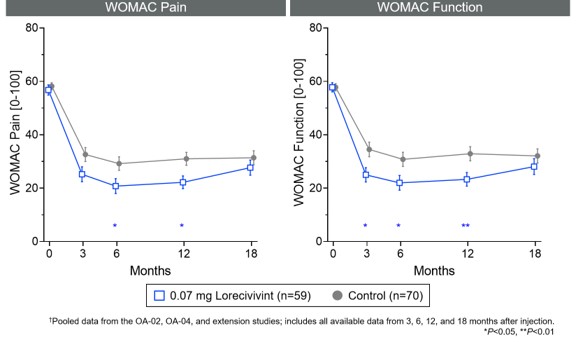
Results: Of 703 subjects, 119 (17%) subjects discontinued prior to study termination. Subject characteristics are shown in Table 1. The safety analysis set included 495 LOR-treated subjects and 208 control subjects (Table 2). Four AEs in 3 (0.6%) subjects across LOR groups were considered related to the study drug; no subjects withdrew from the study due to a treatment-related AE. Incidence was similar between LOR and PBO groups. Sixty-eight SAEs in 38 (5.4%) subjects were reported with none considered related to treatment by investigators. One death occurred in the control group. Post hoc efficacy analyses demonstrated that subjects in the 0.07 mg LOR group (n=59) showed greater mean improvements from baseline in both WOMAC Pain and Function at 6 (Pain: -8.16, 95% CI [-15.60, -0.71], P=0.032; Function: -9.47 [-17.09, -1.84], P=0.015) and 12 (Pain: -8.51 [-15.17, -1.85], P=0.013; Function: -9.62 [-16.83, -2.42], P=0.009) months vs. subjects in the control group (n=70) (Figure 1). No mJSW progression was observed in any group over 18 months. Limitations included using subjects (completers) more likely to be responders and not controlling for other treatments in the extension study period.
Conclusion: LOR appeared safe and well tolerated. Efficacy analyses on the described subset of completer subjects demonstrated durable symptom improvements in WOMAC Pain and Function for up to at least 12 months vs. controls.
To cite this abstract in AMA style:
Simsek I, Swearingen C, Ghandehari H, Kennedy S, Tambiah J, Yazici Y, Skrepnik N. A Multicenter, Observational, Extension Study Evaluating the Safety, Tolerability, and Efficacy of a Single Lorecivivint Injection in Knee OA Subjects [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/a-multicenter-observational-extension-study-evaluating-the-safety-tolerability-and-efficacy-of-a-single-lorecivivint-injection-in-knee-oa-subjects/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-multicenter-observational-extension-study-evaluating-the-safety-tolerability-and-efficacy-of-a-single-lorecivivint-injection-in-knee-oa-subjects/