Session Information
Date: Sunday, November 5, 2017
Title: Osteoporosis and Metabolic Bone Disease – Clinical Aspects and Pathogenesis Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Four clinical trials have separately shown greater BMD gains with transitioning to denosumab (DMAb) compared with continuing on bisphosphonates (BP) in subjects previously treated with oral BPs (Kendler JBMR 2010; Recknor Obstet Gynecol 2013; Roux Bone 2014; Miller JCEM 2016). The aim of this meta-analysis was to improve estimates of effect size and provide an integrated assessment of safety/efficacy of DMAb vs BPs with different dosing regimens (weekly, monthly, yearly) and administration routes (oral, intravenous), over 12 months in postmenopausal women pretreated with oral BPs.
Methods: Data were pooled from 4 randomized studies in postmenopausal women with low bone mass or osteoporosis, aged ≥55 years, and pretreated with oral BPs, who were randomized 1:1 to DMAb (60mg every 6 months) or an oral (alendronate 70mg weekly, ibandronate 150mg monthly, risedronate 150mg monthly) or intravenous (zoledronic acid 5mg yearly) BP for 12 months. Percentage (%) change from baseline (BL) in BMD at the lumbar spine, total hip, femoral neck, and 1/3 radius (assessed in 2 studies) at month 12; % change from BL in serum C-terminal telopeptide of type I collagen (sCTX, in a subset of 1058 subjects) at 1, 6, and 12 months (in 2 of the studies); and safety were assessed. Fractures were collected as adverse events (AEs) and not adjudicated.
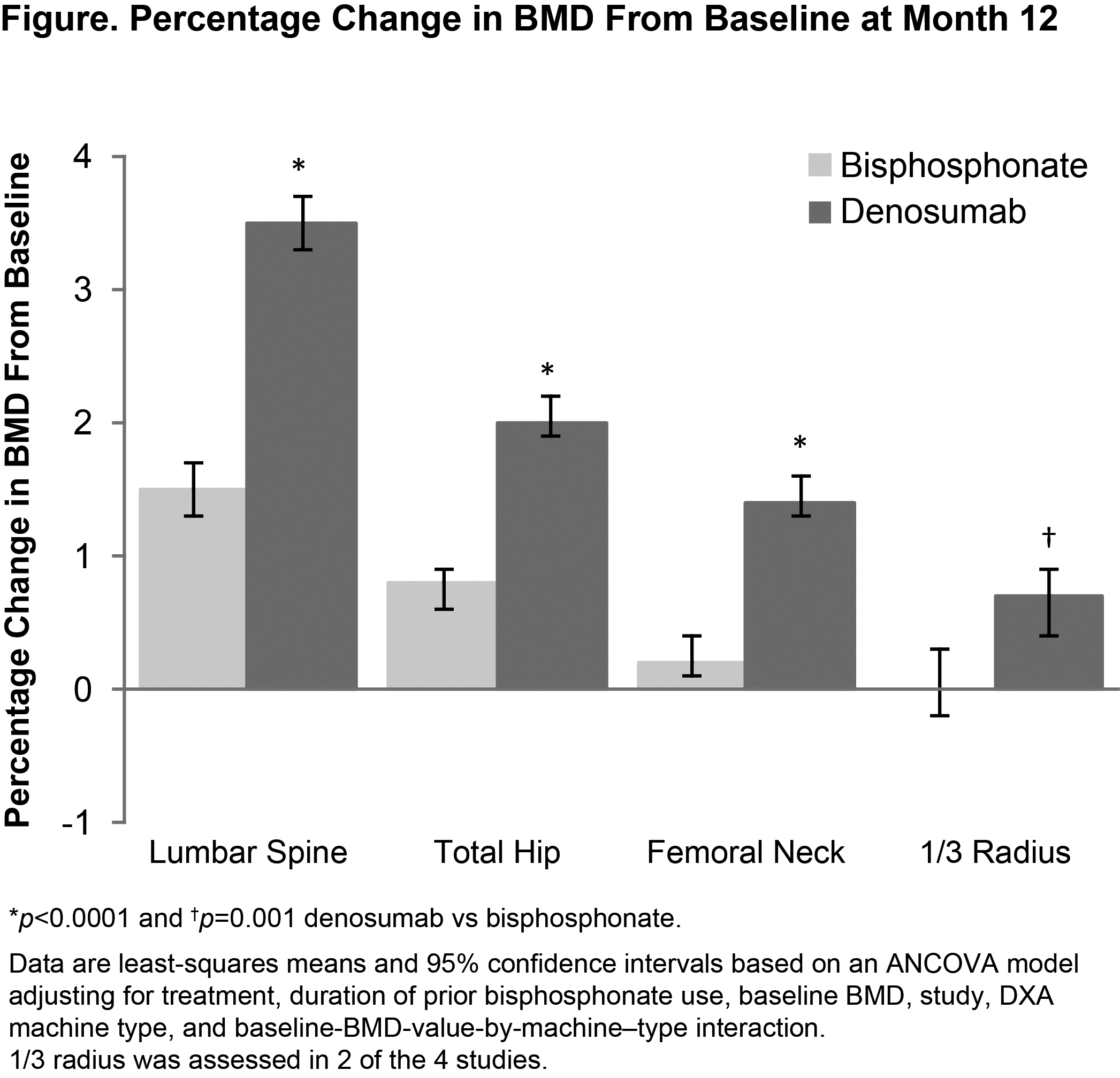
Results: A total of 2850 subjects were included (1426 DMAb; 1424 BP). Mean (SD) age was 68 (8) years, mean (SD) lumbar spine BMD T-score was –2.5 (1.0), and mean (SD) duration of prior oral BP use was 3.8 (3.6) years. BMD % change from BL at month 12 was significantly greater with DMAb vs BPs at all measured skeletal sites (Figure) and independent of length of prior BP use (<2 or ≥2 years) at all sites measured (except for 1/3 radius for those with <2 years of prior BP use). Median sCTX % decrease from BL was greater with DMAb than BPs at months 1 (–58% vs –12%), 6 (–36% vs –14%), and 12 (–26% vs 8%; all p<0.0001). Overall AEs/serious AEs were similar between groups. There were no cases of osteonecrosis of the jaw. Three events consistent with the definition of atypical femoral fracture were observed (2 DMAb; 1 BP). Osteoporosis-related fractures were reported in 47 (3.3%) DMAb and 43 (3.1%) BP subjects.
Conclusion: This integrated assessment shows greater clinical benefit with increases in BMD and reductions in bone turnover and similar safety profile in transitioning from oral BPs to DMAb, compared with continuing on or cycling through the same therapeutic class (from one BP to another).
To cite this abstract in AMA style:
Miller PD, Pannacciulli N, Malouf J, Singer A, Czerwinski E, Bone H, Wang C, Wagman RB, Brown J. A Meta-Analysis of 4 Clinical Trials of Denosumab Compared with Bisphosphonates in Postmenopausal Women Previously Treated with Oral Bisphosphonates [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/a-meta-analysis-of-4-clinical-trials-of-denosumab-compared-with-bisphosphonates-in-postmenopausal-women-previously-treated-with-oral-bisphosphonates/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-meta-analysis-of-4-clinical-trials-of-denosumab-compared-with-bisphosphonates-in-postmenopausal-women-previously-treated-with-oral-bisphosphonates/