Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Mediation modeling is used to determine mechanisms underlying the relationship between independent and dependent variables via other explanatory variables (“mediators”). Fatigue, pain, and morning stiffness are common ankylosing spondylitis (AS) symptoms that can contribute to decreased functional capacity and health-related quality of life (HRQoL) in AS patients. This study aimed to assess how tofacitinib affects HRQoL improvement directly and indirectly (via symptom improvement) in patients with AS.
Methods: Mediation modeling was used to analyze pooled data from phase 2 (NCT01786668) and phase 3 (NCT03502616) studies of tofacitinib. The initial mediation model is depicted by Figures 1A and 1B; superimposition of both figures represents the full mediation model. The independent variable was treatment (tofacitinib 5 mg BID vs placebo) and the dependent variables were the SF-36 domains. Mediators included in the model were CRP, stiffness (represented by the mean of BASDAI questions 5 and 6), mobility (measured by BASMI), fatigue (measured by FACIT-Fatigue total score), and pain (latent factor measured by two numerical rating scales [0-10] for nocturnal pain and total back pain). All available data from week 12 (phase 2 trial) and week 16 (phase 3 trial) were used. Based on the initial model results, the final model was respecified to include only paths that were statistically significant (p< 0.05) and/or meaningful (standardized path coefficient >0.1). The relative contribution of each path from treatment to SF-36 domain was reported in terms of percentages of the total effect of tofacitinib on an SF-36 domain.
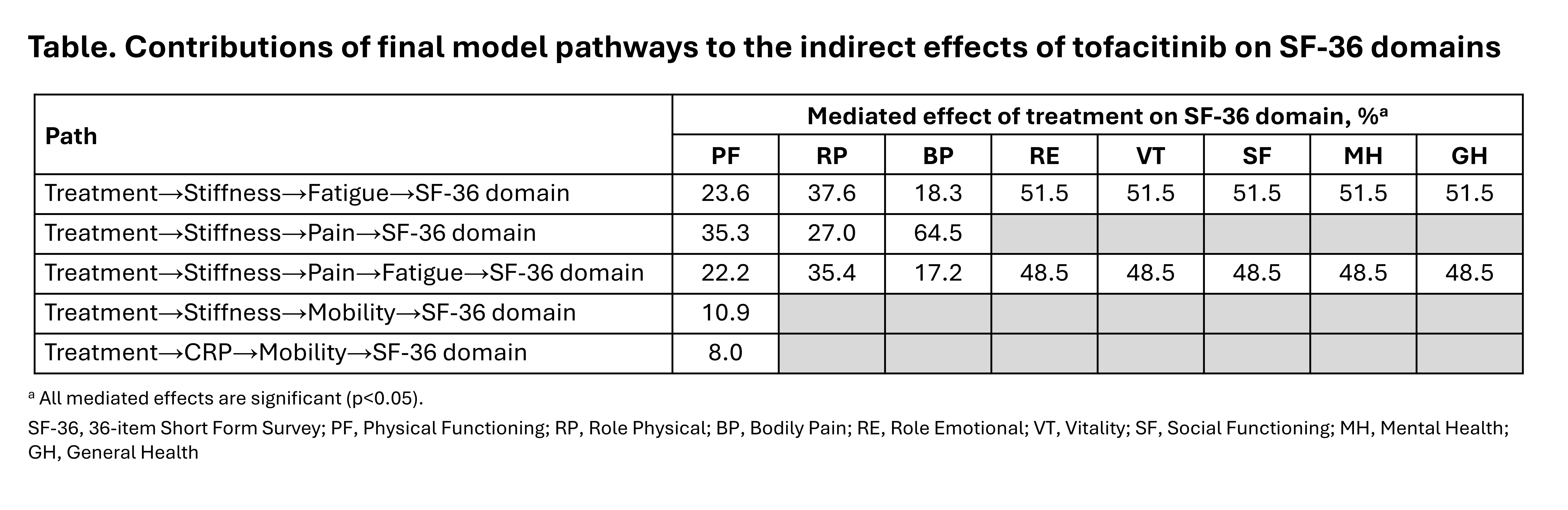
Results: Data from 360 patients were included. The initial model demonstrated that tofacitinib treatment affects SF-36 mainly indirectly. In the final model (Figure 2), improvements in HRQoL were collectively mediated through five indirect pathways: (1) treatment→stiffness→pain→SF-36 domain, (2) treatment→stiffness→fatigue→SF-36 domain, (3) treatment→stiffness→pain→fatigue→SF-36 domain, (4) treatment→stiffness→mobility→SF-36 domain, and (5) treatment→CRP→mobility→SF-36 domain. For the Physical Functioning and Bodily Pain domains, paths via changes in stiffness and pain had the largest effect, mediating 35.3% (p< .0001) and 64.5% (p< .0001) of the tofacitinib treatment effect (Table). For remaining domains, paths via changes in stiffness and fatigue had the largest effect, mediating 37.6% (p=.0002) of the tofacitinib effect on the Role Physical domain and 51.5% (p< .0001) for the other domains. The effect of treatment mediated via changes in stiffness and mobility (10.9%, p< .0001) and via CRP and mobility (8.0%, p=.003) was present only for the Physical Functioning domain.
Conclusion: In patients with AS, the effect of tofacitinib treatment on HRQoL as measured by SF-36 domains was fully mediated via changes in fatigue, pain, stiffness, CRP, and mobility. Tofacitinib reduces the signs and symptoms of AS, which improves HRQoL. By closely assessing the signs and symptoms of AS, physicians can better improve patients’ HRQoL and hence patient care and treatment. The results also suggest that HRQoL measurements may play an important role in assessing the signs and symptoms of AS.
To cite this abstract in AMA style:
Baraliakos X, Bushmakin A, Cappelleri J, Nikitopoulou E, Ling Y, Zayed M, Cicci J. A Mediation Analysis of Tofacitinib’s Effects on SF-36: The Roles of Fatigue, Pain, Stiffness, and Mobility in Ankylosing Spondylitis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-mediation-analysis-of-tofacitinibs-effects-on-sf-36-the-roles-of-fatigue-pain-stiffness-and-mobility-in-ankylosing-spondylitis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-mediation-analysis-of-tofacitinibs-effects-on-sf-36-the-roles-of-fatigue-pain-stiffness-and-mobility-in-ankylosing-spondylitis/

.jpg)
.jpg)