Session Information
Date: Sunday, November 17, 2024
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Bile Salt-Stimulated Lipase (BSSL) is a lipolytic enzyme secreted by the lactating mammary gland and exocrine pancreas. Elevated plasma levels of BSSL have been associated with disease activity in patients with RA, JIA and PsA, indicating that it may have a role in inflammation. This hypothesis has been verified in rodent arthritis models including BSSL knockout mice.
SOL-116 is a humanized monoclonal antibody against BSSL, currently in early clinical development for the treatment of RA. SOL-116 blocks BSSL-monocyte interaction thereby preventing the migration of inflammatory cells. Here we report results from a randomized, double-blind, placebo-controlled, first-in-human clinical trial of single and multiple doses of SOL-116 in 3 parts.
Methods: In the single ascending dose (SAD) part, 40 adult healthy subjects were randomized to receive SOL-116 or matching placebo (3:1), administered as SC injection in 5 cohorts. Each dose escalation was preceded by a blinded safety assessment and interim pharmacokinetic (PK) analysis by a safety review committee. In the multiple dosing (MD) part, a single cohort of 8 adult, healthy subjects received 4 doses of SOL-116 or matching placebo (3:1), 4 weeks apart.
The primary endpoint was safety and tolerability of SOL-116; secondary endpoints were PK parameters and markers of immunogenicity. Exploratory endpoints included pre- and post-dose plasma BSSL concentration and inflammatory cytokines.
The study also includes a cohort in which RA patients receive a single dose of SOL-116 or matching placebo (ongoing).
All 3 parts of the trial included a follow up 90 days after the last dose.
Results: Five SAD cohorts of 8 healthy subjects successfully completed the study, receiving single doses of 0.075, 0.225, 0.675, 2.025 and 6.075 mg/kg SOL-116, respectively (n=30), or placebo (n=10).
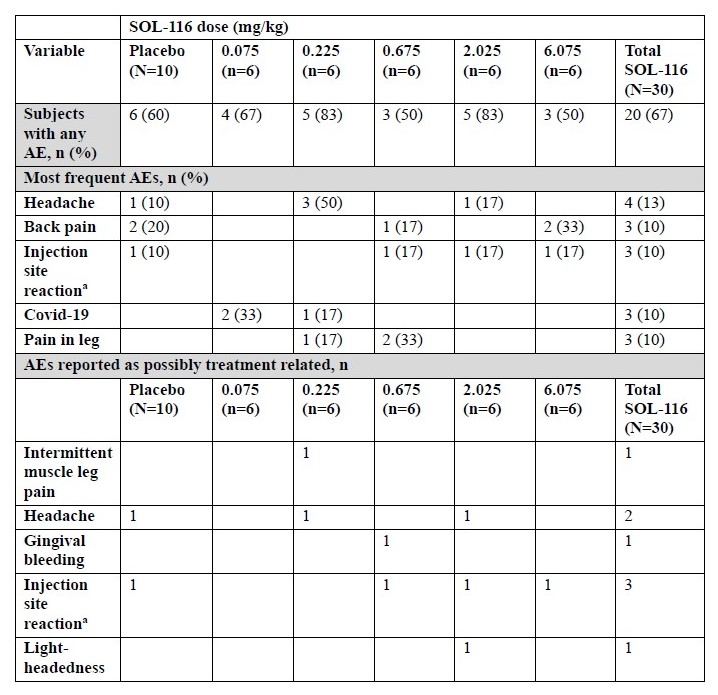
SOL-116 was well tolerated across all 5 SAD cohorts. No SAEs were reported, and AEs were mostly mild and transient. Only 8/36 AEs reported by 30 subjects receiving SOL-116 were deemed possibly related to SOL-116 (Table 1).
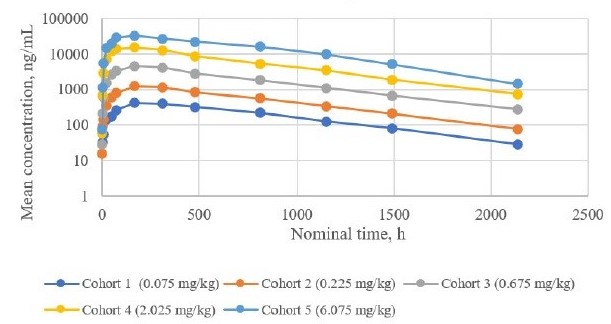
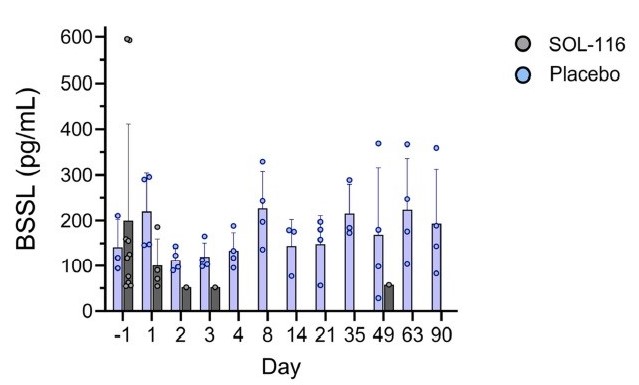
The PK profile of SOL-116 in the SAD cohorts was favorable and consistent with preclinical results. Serum SOL-116 exposure was approximately dose-proportional across the SAD cohorts, with the maximum dose of 6.075 mg/kg resulting in a Cmax of 34.1 µg/mL (Figure 1). Mean Tmax ranged from 5 to 9 days with a mean terminal elimination half-life of approximately 20 days. In subjects receiving SOL-116, BSSL plasma levels were essentially undetectable from day 3 after SOL-116 administration until day 90 post-dose (Figure 2).
In the MD part, a single cohort of 8 healthy subjects each received 4 SC doses of 3.0 mg/kg SOL-116 (n=6) or placebo (n=2), 4 weeks apart. Subjects had few AES and there were no SAEs in subjects administered SOL-116. The PK parameters in the MD part were consistent with the SAD part. A linear dose versus systemic exposure relationship was observed.
Conclusion: SOL-116 was well tolerated, with robust and predictable PK and no induction of ADAs following single and multiple dosing in healthy subjects. Single doses of SOL-116 effectively eliminated circulating BSSL. These findings strongly support the continued clinical development of SOL-116.
a. Comprising pain at injection site, hematoma at injection site, and redness at injection site.
To cite this abstract in AMA style:
Franck-Larsson K, Lindquist S, Wennerholm A, Kolmert J, Wanderoy M, Hernell O. A First-in-Human Clinical Trial Evaluating the Safety and Pharmacokinetics of SOL-116, a Novel Humanized Monoclonal Antibody Targeting Bile Salt-Stimulated Lipase for the Treatment of RA [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-first-in-human-clinical-trial-evaluating-the-safety-and-pharmacokinetics-of-sol-116-a-novel-humanized-monoclonal-antibody-targeting-bile-salt-stimulated-lipase-for-the-treatment-of-ra/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-first-in-human-clinical-trial-evaluating-the-safety-and-pharmacokinetics-of-sol-116-a-novel-humanized-monoclonal-antibody-targeting-bile-salt-stimulated-lipase-for-the-treatment-of-ra/