Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Fibromyalgia (FM) generally is easily recognized, but a diagnosis may be difficult, particularly in patients with secondary FM who have other primary diagnoses. Criteria for FM initially were reported in 1990, and revised in 2010, 2011 and 2016. The 2 most recent criteria are based entirely on patient self-report, which is not collected in most routine clinical care, as multiple patient questionnaires are not feasible in busy clinical settings. MDHAQ/RAPID3 (multi-dimensional health assessment questionnaire/routine assessment of patient index data) is informative in all diseases studied. Cumulative indices based on MDHAQ scales known as FAST3 (fibromyalgia assessment screening tool3) recognize FM at levels of agreement with revised FM criteria of >80% and correlations of >0.80, p< 0.001), and might provide clues to recognize FM in patients with non-rheumatic diseases, as most components of the MDHAQ (as well as the HAQ) appear generic rather than disease-specific, other than a RADAI self-report painful joint count. Therefore, we analyzed a FAST3-nJC (no RADAI JC) index versus 2011 FM criteria as a reference standard, and compared to other FAST3 indices which include a painful RADAI JC.
Methods: All patients with all diagnoses complete an MDHAQ at all visits in routine care at one setting. The self-report questionnaire to recognize the 2011 FM Criteria was added over a 6-month period to be completed by consecutive patients. The MDHAQ includes 0-10 scores for physical function, and pain and patient global visual analog scales (VAS), compiled into 0-30 RAPID3, as well as a 0–10 fatigue VAS, 0–54 self-report painful joint count, and 0–60 symptom checklist. Each MDHAQ scale was analyzed for agreement with FM Criteria according to receiver-operator-characteristic (ROC) curves for area under the curve (AUC). Optimal cut points for each measure were identified, based on specificity and sensitivity, to develop optimal cumulative indices for clues to FM versus the 2011 Criteria as “gold standards.”
Results: Among 502 patients with complete data, primary ICD-10 diagnoses were FM in 49, OA in 74, RA in 78, SLE 88 and other rheumatic diseases in 213. Primary or secondary FM was identified in 131 (26%) who met 2011 FM criteria. The 4 MDHAQ scales with the highest AUC vs FM Criteria (0.829-0.889) and optimal cut-points, were symptom checklist 16/60, RADAI JC 16/54, fatigue VAS 6/10, and pain VAS 6/10. FAST3 0-3 measures were calculated as 0 or 1 based on cut-points for each measure, FM=2 or 3: FAST3-P= symptom checklist+RADAI JC+pain VAS; FAST3-F=symptom checklist+RADAI JC+fatigue VAS; FAST3nJC=symptom checklist+painVAS+fatigue VAS(no RADAI). All FAST3 indices agreed with FM Criteria >80% and kappas were >0.58, indicating good agreement (Table). Lowest agreement was seen for FAST3nJC, expected as FM criteria include a self-report painful joint count, but differences are small.
Conclusion: FAST3nJC had slightly lesser agreement with 2011 and 2016 FM criteria than FAST3-P and FAST3-F but would appear satisfactory to provide clues to recognize FM in patients with non-rheumatic diseases, although a diagnosis of FM ultimately is made by a physician.
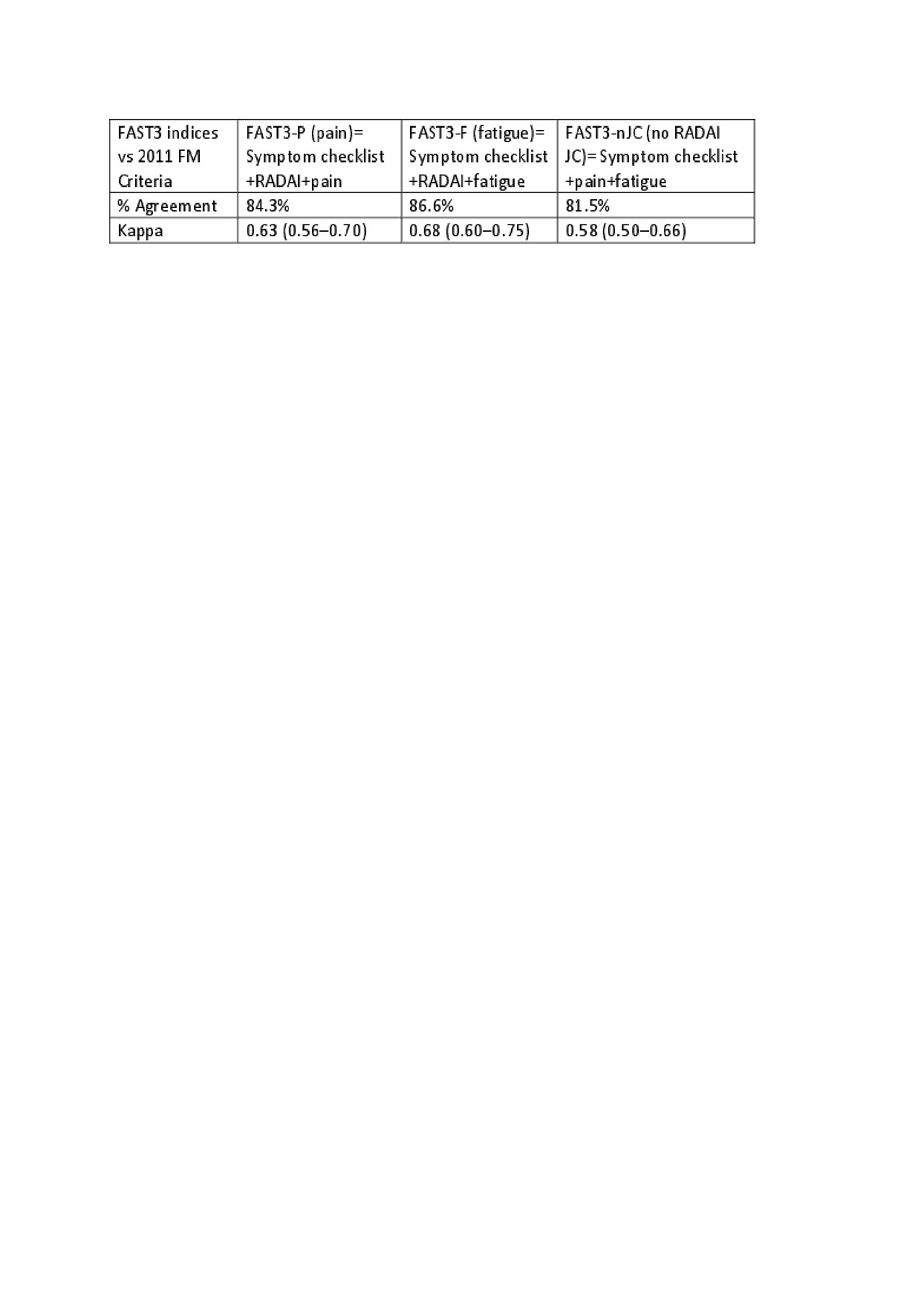
Table FAST3 indices vs 2011 FM Criteria
To cite this abstract in AMA style:
Pincus T, Castrejon I, Block J. A Fibromyalgia Assessment Screening Tool on a Multidimensional Health Assessment Questionnaire (MDHAQ) Which Does Not Include a Self-Report RADAI Painful Joint Count (RADAI), FAST3nJC, Recognizes Fibromyalgia Similarly to Other FAST3 Indices Which Include a RADAI [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-fibromyalgia-assessment-screening-tool-on-a-multidimensional-health-assessment-questionnaire-mdhaq-which-does-not-include-a-self-report-radai-painful-joint-count-radai-fast3njc-recognizes-fibr/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-fibromyalgia-assessment-screening-tool-on-a-multidimensional-health-assessment-questionnaire-mdhaq-which-does-not-include-a-self-report-radai-painful-joint-count-radai-fast3njc-recognizes-fibr/
