Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Women of child bearing age with autoimmune diseases are often
prescribed teratogenic medications. Contraceptive compliance
in this group of patients has been shown to be low. We conducted a quality
improvement project to increase contraceptive compliance in eligible patients
in the Duke Rheumatology outpatient clinic.
Methods: We reviewed charts of 18-45
year old women on teratogenic medications (methotrexate,
leflunomide, mycophenolate
and cyclophosphamide) to evaluate baseline contraception compliance in our
practice. Contraceptive compliance was measured by reviewing documentation of
1) contraception type 2) counseling regarding contraceptives and 3) further
intervention after counseling. Using
Plan-Do-Study-Act (PDSA) methodology, we implemented a series of changes in our
clinic based on periodic input from stakeholders. Interventions centered on inter-professional
provider education, modification of the electronic medical record (EMR) templates,
periodic provider reminders, in-clinic reminder sheets, and frequent feedback to
providers on performance. During each monthly PDSA cycle, one week of patients’
charts were reviewed for performance measurement.
Results: At baseline, eligible patients
(n = 181 out of 3003 reviewed charts) rate of documentation for contraception
usage was 46%, counseling was 33%, and interventions after counseling occurred
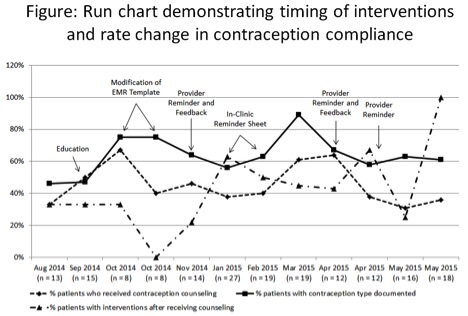
in 33%. As shown in the run chart, initial education led to a non-sustained improvement
in provider compliance. Modification of the EMR template improved provider
compliance, but note cloning inhibited extended improvement. In-clinic reminder
sheets, frequent feedback and periodic stakeholder discussions had a mixed
impact. The rate of contraception type documented at the end of the project was
61%, contraception counseling was 67%, and further intervention performed after
counseling was 100%. During this project, one unintentional pregnancy that led
to miscarriage occurred in a patient on teratogenic
medication not using contraception.
Conclusion: Overall, documentation of contraceptive
type, counseling and intervention increased, but room for improvement remains. Every woman taking teratogenic
medication should be using contraception, and even during a quality improvement
effort in a single clinic, 100% compliance was not achieved. Therefore more creative solutions are
needed, whether EMR-based alerts, provider incentives, personalized feedback, or
a combination of interventions. Discovery of a miscarriage in a patient
receiving one of these medications highlights the importance of the continued
discussions with our patients.
To cite this abstract in AMA style:
Wells M, Lackey V, Peart E, Holdgate N, Mohammad S, Balevic S, Sadun R, Criscione-Schreiber LG, Clowse MEB, Yanamadala M. A Fellow Led Quality Improvement Project for Improving Contraceptive Compliance in Women Receiving Teratogenic Medications [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/a-fellow-led-quality-improvement-project-for-improving-contraceptive-compliance-in-women-receiving-teratogenic-medications/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-fellow-led-quality-improvement-project-for-improving-contraceptive-compliance-in-women-receiving-teratogenic-medications/