Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Avacopan, a C5a receptor inhibitor, is used as an adjunctive treatment for ANCA-associated vasculitis. Previous studies have highlighted its efficacy in reducing glucocorticoid use and improving renal outcomes. However, concerns regarding its safety profile, particularly hepatotoxicity and serious infections, remain significant. This study aims to investigate the incidence and characteristics of adverse events associated with Avacopan using the FDA Adverse Event Reporting System (FAERS) database. By analyzing real-world pharmacovigilance data, this study seeks to provide a comprehensive assessment of the risks posed by Avacopan, thereby informing clinical practice and enhancing patient safety.
Methods: The FAERS database was searched for adverse events related to avacopan. Data were reported as frequencies, proportional reporting ratio (PRR), and as reporting odds ratios (ROR) with a 95% confidence interval (95% CI).
Results: We analyzed 30,179,725 adverse event reports from the FAERS database. A total of 3,658 reports were associated with the use of avacopan. 39.15% of patients were females, whereas 32.34% had an unspecified sex. The most common group of adverse reactions was generalized side effects (1,103), followed by infections (675) and gastrointestinal disorders (635) (Figure 1). Patients on avacopan had a higher ROR of getting fatigue compared to all other drug users (ROR=2.422; 95% CI: 2.101-2.791). In addition, gastrointestinal disorders, including diarrhea and nausea, were higher among avacopan users compared to other drug users (ROR= 2.611 and 2.028, respectively). Drug-induced liver injury was reported in 52 patients on avacopan, and patients who were taking the medications were significantly more likely to get this complication (ROR=17.59; 95% CI: 13.37-23.14) (Table 1).
Conclusion: In this large pharmacovigilance analysis, avacopan use was associated with a distinct adverse event profile, most notably fatigue, gastrointestinal symptoms, and a markedly increased reporting odds of drug-induced liver injury. While these findings underscore known risks observed in clinical trials, the elevated RORs for hepatic events warrant continued surveillance and caution in clinical use. Real-world safety data such as these are critical for guiding risk mitigation strategies and optimizing patient outcomes in ANCA-associated vasculitis.
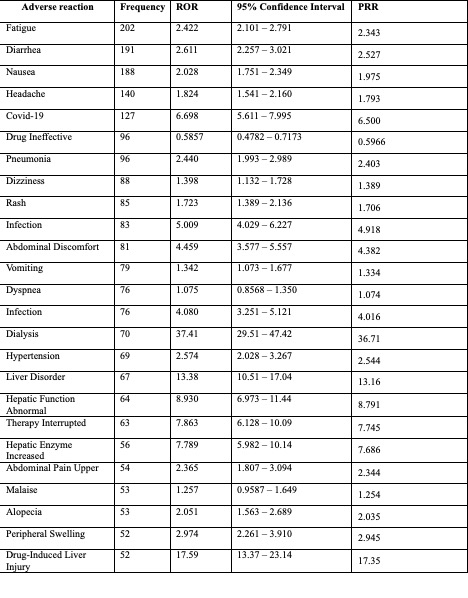 Figure 1. Frequency of adverse events
Figure 1. Frequency of adverse events
.jpg) Table 1. Reported Odds Ratio (ROR) and Proportional Reporting Ratio (PRR) of Adverse Events
Table 1. Reported Odds Ratio (ROR) and Proportional Reporting Ratio (PRR) of Adverse Events
To cite this abstract in AMA style:
Cooper S, Khanfar A, al-mabrouk Y, Hamdan O, Ocon A. A Disproportionality Analysis of FDA Adverse Event Reporting System (FAERS) Events for Avacopan [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-disproportionality-analysis-of-fda-adverse-event-reporting-system-faers-events-for-avacopan/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-disproportionality-analysis-of-fda-adverse-event-reporting-system-faers-events-for-avacopan/
