Session Information
Date: Tuesday, November 19, 2024
Title: Abstracts: Miscellaneous Rheumatic & Inflammatory Diseases II
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: VEXAS is a newly described “haemato-autoinflammatory” condition resulting from somatic mutations in the UBA-1 gene. By integrating genetics, cytology and imaging, this work seeks to advance a diagnostic pathway to better characterize VEXAS patients from the outset.
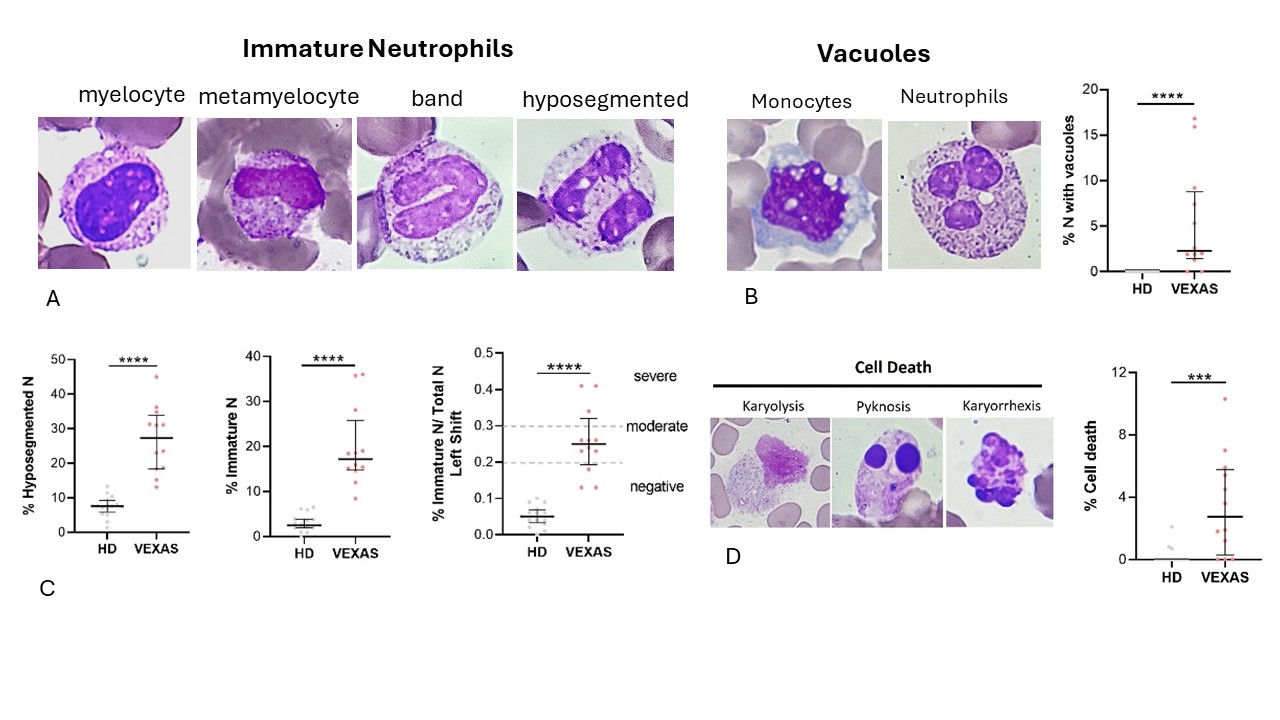
Methods: Fourteen consecutive males (median age 74.5, IQR 8.5) with suspected VEXAS syndrome and 16 healthy donors (HDs) were gathered. Whole blood from patients was collected for exome analysis (HiSeq2500 Illumina Sequencer) to assess the potential carriage of UBA-1 mutations. In parallel, peripheral blood cells were examined. May-Grünwald Giemsa staining was used to study the cellular morphology, including cytoplasm, granules, and vacuoles, and to perform a cytogenic evaluation of leukocytes. Interleukin (IL)-1β, TNFα, IL-1α, IL-18 and IL-8 were measured in plasma using commercially available enzyme-linked immunosorbent assay (ELISA) kits. As a preliminary work-up, positron-emission tomography/computed tomography (PET-CT) scans were executed; images were analysed drawing regions of interest (ROI), and a semi-quantitative analysis was performed for each scan by fixing the Standardized Uptake Values (SUVs).
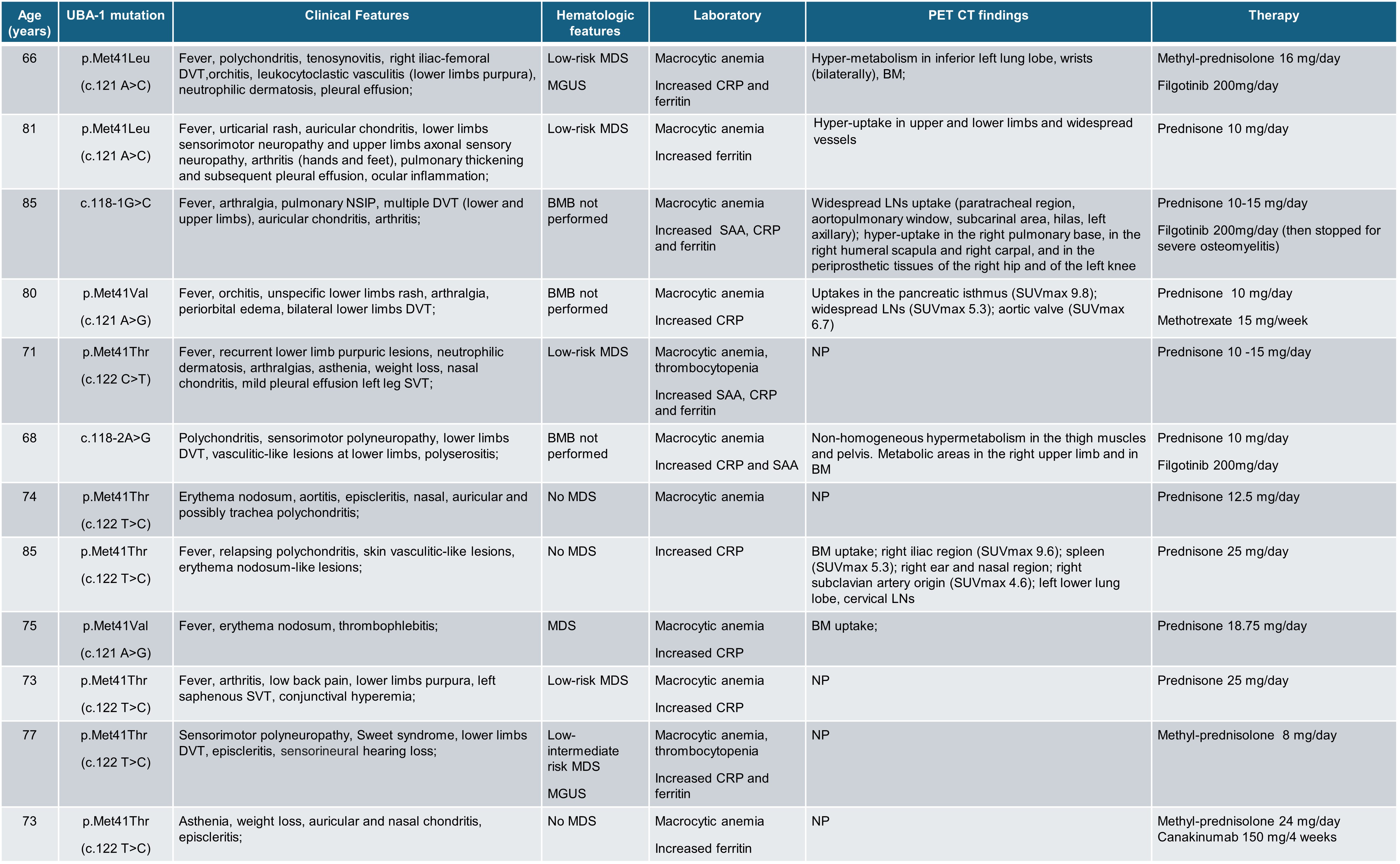
Results: Twelve patients (86%) had mutations in the UBA-1 gene. Table 1 shows the characteristics of the patients with VEXAS. At cytological evaluation (Figure 1), hyposegmented and immature neutrophils (band cells, metamyelocytes, and myelocytes) were higher in VEXAS than HDs (p< 0.0001). A moderate shift to the left (0.2 – 0.29) and a severe shift to the left (≥ 0.3) were observed in 3 (25%) and 7 (58%) VEXAS patients, respectively; the I/T index was higher in VEXAS than HDs (p< 0.0001). Vacuoles were observed in monocytes and neutrophils of 10/12 (83%) patients and were absent in HDs (p< 0.0001). Although not significant, the percentage of neutrophils and immature neutrophils was higher in patients with the Met41Val mutation compared to the other variants. A significant difference was observed in the cell death rate between VEXAS and controls (p< 0.001). Regarding cytokines, no differences in the IL-1β levels were found between HDs and VEXAS. The levels of IL-18 (p< 0.0001), IL-1α (p< 0.05), TNFα (p< 0.01) and IL-8 (p< 0.05) were higher in VEXAS. Patients carrying the Met41Val variation had higher IL-1β, IL-18, IL-1α and IL-8 levels than those with other known mutations. The percentage of cell death correlated with IL-1β (r=0.840, p =0.001) and IL-8 (r=0.758, =0.006). Finally, IL-8 levels correlated with the vacuoles (r= 0.732, p=0.009) and micronuclei rates (r=0.777, p=0.005). PET-CT scans were performed in 7/12 (58%) patients showing diffuse hyper-uptakes mainly in bone marrow (57%) and lymph nodes (43%) (Table 1).
Conclusion: Cytological evaluation appears to be a rapid and non-invasive way of detecting abnormalities consistent with the suspected diagnosis while waiting for genetics, or as an alternative to bone marrow biopsy. To facilitate a prompt treatment start, the PET-CT scan may be helpful in ruling out mimicking pathologies such as solid cancer, lymphoproliferative diseases, and other poly/monogenic autoinflammatory conditions.
Legend: BM: bone marrow; BMB: bone marrow biopsy; CRP: C-reactive protein; DVT: deep vein thrombosis; LNs: lymphnodes; MDS: myelodysplastic syndrome; MGUS: monoclonal gammopathy of unknown significance; NP= not performed; NSIP: non-specific interstitial pneumonia; SAA: serum amyloid A; SUV: standardized uptake value; SVT: superficial vein thrombosis;
To cite this abstract in AMA style:
Bindoli S, Baggio C, Padoan R, Bixio R, Andrea d, Ramonda R, Sfriso P. A Comprehensive Approach Utilizing a Combination of Genetics, Cytological Analysis, and Imaging Techniques to Speed up the Diagnostic Process in VEXAS Patients [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-comprehensive-approach-utilizing-a-combination-of-genetics-cytological-analysis-and-imaging-techniques-to-speed-up-the-diagnostic-process-in-vexas-patients/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-comprehensive-approach-utilizing-a-combination-of-genetics-cytological-analysis-and-imaging-techniques-to-speed-up-the-diagnostic-process-in-vexas-patients/