Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Filgotinib (FIL), an oral, selective, Janus Kinase 1 (JAK1) inhibitor was effective in Phase 3 studies of active RA in patients (pts) with inadequate response or intolerance to biologic DMARDs (bDMARD-IR; FINCH-2 ClinicalTrials.gov Identifier: NCT02873936). RA pts with high IFN scores have shown a diminished clinical response to first-line treatments (Rodríguez-Carrio et al. 2018; Cooles FAH et al. 2018) and differential responses to second-line treatments (Mavragani et al. 2010; Thurlings R et al. 2010). Baseline IFN scores have been associated with a week 12 FIL-specific ACR-N response in the DARWIN Phase 2 trials (Taylor et al. 2018). The value of baseline gene expression for predicting therapeutic response to FIL in a bDMARD experienced RA population was explored in an RNA sequencing study using FINCH-2 pts.
Methods: Blood samples from RA pts in FINCH-2 on either a stable dose of MTX and placebo (n = 144), or once daily FIL 100 mg (n = 151), or 200 mg (n = 143) were analyzed at baseline and weeks 4 and 12. To evaluate the relationship between baseline IFN activity and change in disease activity after 12 weeks, we tested two IFN-based signatures: a 4-gene panel (Rodríguez-Carrio et al. 2018) and a FINCH-2 composite score derived from IFN response genes and clinical assay results. The association of these two signatures at baseline with the week 12 change in disease activity score 28 CRP (DAS28-CRP) or ACR-N response were evaluated using a linear regression model. The main effect of signature activity on clinical response represents a prognostic effect (i.e. independent of treatment), while a multiplicative interaction between signature activity and FIL is predictive. A linear mixed-effects model determined the FIL-specific change in signature activity across 2 time-points post-baseline (weeks 4 and 12).
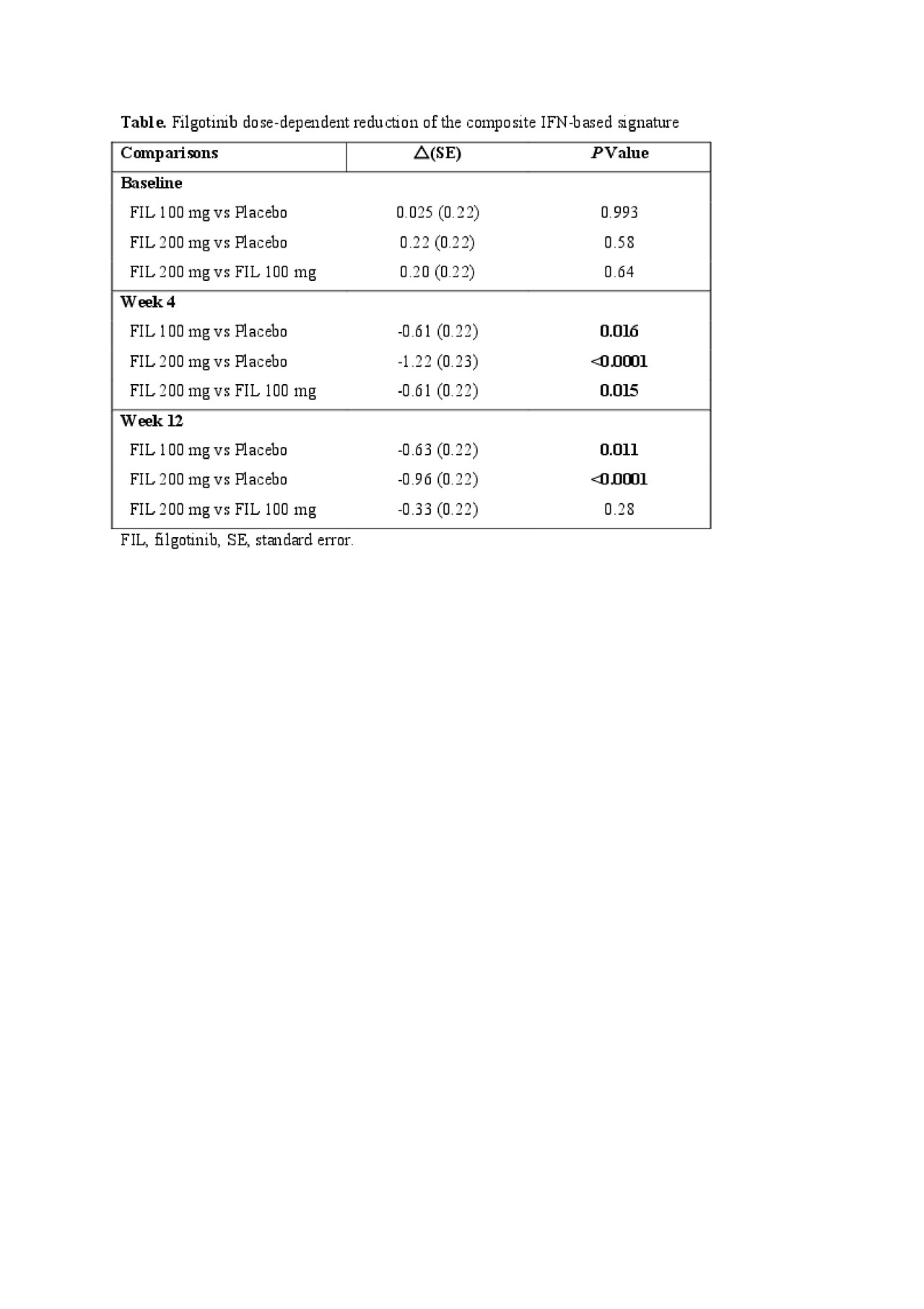
Results: Baseline Rodriguez-Carrio IFN signature score showed no significant association with week 12 clinical response (P > 0.05 for all comparisons). In contrast, the baseline FINCH-2 derived composite IFN-based signature was significantly associated with an increase of DAS28-CRP after 12 weeks (P = 0.0026), independent of treatment. The composite IFN-based signature score was significantly associated with a FIL-specific improvement in DAS28-CRP (FIL 100 mg, P = 0.0045; FIL 200 mg, P = 0.0005) and ACR-N responses (FIL 100 mg, P = 0.036) after 12 weeks. The composite signature was significantly reduced in a dose-dependent manner after FIL treatment at weeks 4 and 12 (Table).
Conclusion: Expression of 4 IFN-stimulated genes at baseline was not significantly associated with week 12 clinical response in FINCH-2, suggesting that IFN signalling alone is not sufficient to predict response to MTX in a bDMARD experienced population. However, an expanded FINCH‑2 derived composite IFN signature demonstrated a treatment-specific significant association at week 12. Validation of this signature or its sub-components will be performed in the FINCH-1 (MTX-experienced) and FINCH-3 (MTX-naïve) clinical trials, and retrospectively in the DARWIN clinical trials.
To cite this abstract in AMA style:
Taylor P, Downie B, Elboudwarej E, Hawtin R, Mirza A, Liu J. A Composite IFN-Based Signature Is Associated with a Filgotinib-Specific Clinical Response in bDMARD-Experienced Rheumatoid Arthritis Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-composite-ifn-based-signature-is-associated-with-a-filgotinib-specific-clinical-response-in-bdmard-experienced-rheumatoid-arthritis-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-composite-ifn-based-signature-is-associated-with-a-filgotinib-specific-clinical-response-in-bdmard-experienced-rheumatoid-arthritis-patients/