Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Upadacitinib (UPA), a selective JAK1 inhibitor, has shown efficacy in patients with rheumatoid arthritis (RA) when combined with methotrexate (MTX) or other conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs).1,2 However, the efficacy of UPA plus MTX has not been directly compared with UPA plus other csDMARDs.
We compare the efficacy of UPA in combination with MTX versus UPA in combination with other csDMARDs in patients with an inadequate response (IR) to csDMARDs (SELECT-NEXT1) or biologic DMARDs (bDMARDs; SELECT-BEYOND2).
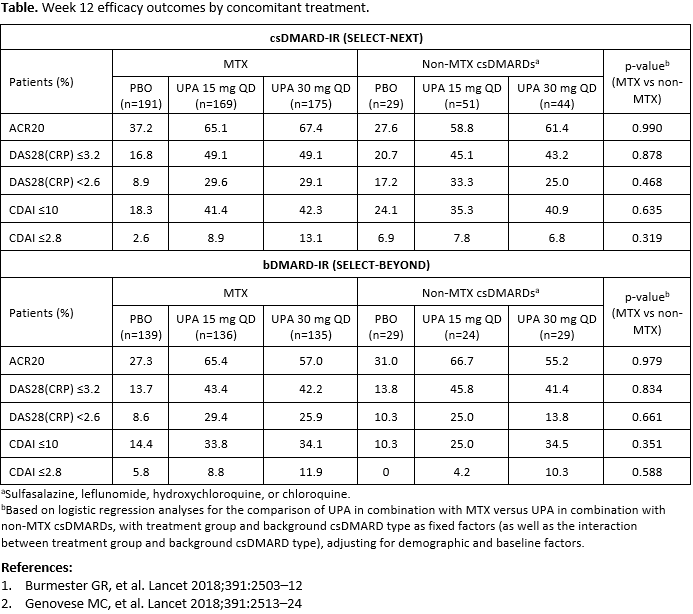
Methods: 661 patients in SELECT-NEXT and 498 patients in SELECT-BEYOND received UPA 15 mg or 30 mg once daily (QD) or placebo (PBO) for 12 weeks; all patients received concomitant csDMARD(s). The primary endpoints for both studies were rates of ACR20 response and DAS28(CRP) ≤3.2. Additional endpoints included DAS28(CRP) < 2.6, CDAI low disease activity (≤10), and CDAI remission (≤2.8). Patients were grouped according to concomitant csDMARD use (MTX vs non-MTX csDMARDs); patients receiving both MTX and a non-MTX csDMARD were included in the MTX group. p-values were calculated based on a logistic regression model with treatment group and type of background csDMARD as fixed factors, adjusting for demographic and baseline factors. p-values from the interaction between treatment group and background csDMARD were also calculated, to assess the consistency of the effects of study treatments for different background csDMARD type. Non-responder imputation was used for missing data.
Results: In SELECT-NEXT and SELECT-BEYOND, 535 and 410 patients, respectively (~80%), were receiving concomitant MTX (mean dose 17 mg/week), and 124 and 82 patients were receiving non-MTX csDMARDs. Demographics and disease characteristics were broadly similar between treatment groups; the majority of patients were female and of white ethnicity, and around half were using oral corticosteroids at baseline. Across all subgroups, the proportion of patients achieving efficacy outcomes was higher with both UPA doses compared with PBO (Table). There were no significant differences between efficacy outcomes with UPA in combination with MTX versus UPA in combination with non-MTX csDMARDs in either patient population. This included ACR20 response as well as low disease activity and remission defined by DAS28(CRP) and CDAI.
Conclusion: In this post hoc analysis, the efficacy of UPA in patients with RA appeared comparable whether administered in combination with MTX or non-MTX csDMARDs.
To cite this abstract in AMA style:
Kremer J, Van den Bosch F, Rubbert-Roth A, Radominski S, Burmester G, Camp H, Meerwein S, Howard M, Song Y, Zhong S, Combe B. A Comparison of Upadacitinib Plus Methotrexate and Upadacitinib Plus Other CsDMARDs in Patients with Rheumatoid Arthritis: An Analysis of Two Phase 3 Studies [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-comparison-of-upadacitinib-plus-methotrexate-and-upadacitinib-plus-other-csdmards-in-patients-with-rheumatoid-arthritis-an-analysis-of-two-phase-3-studies/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-comparison-of-upadacitinib-plus-methotrexate-and-upadacitinib-plus-other-csdmards-in-patients-with-rheumatoid-arthritis-an-analysis-of-two-phase-3-studies/